
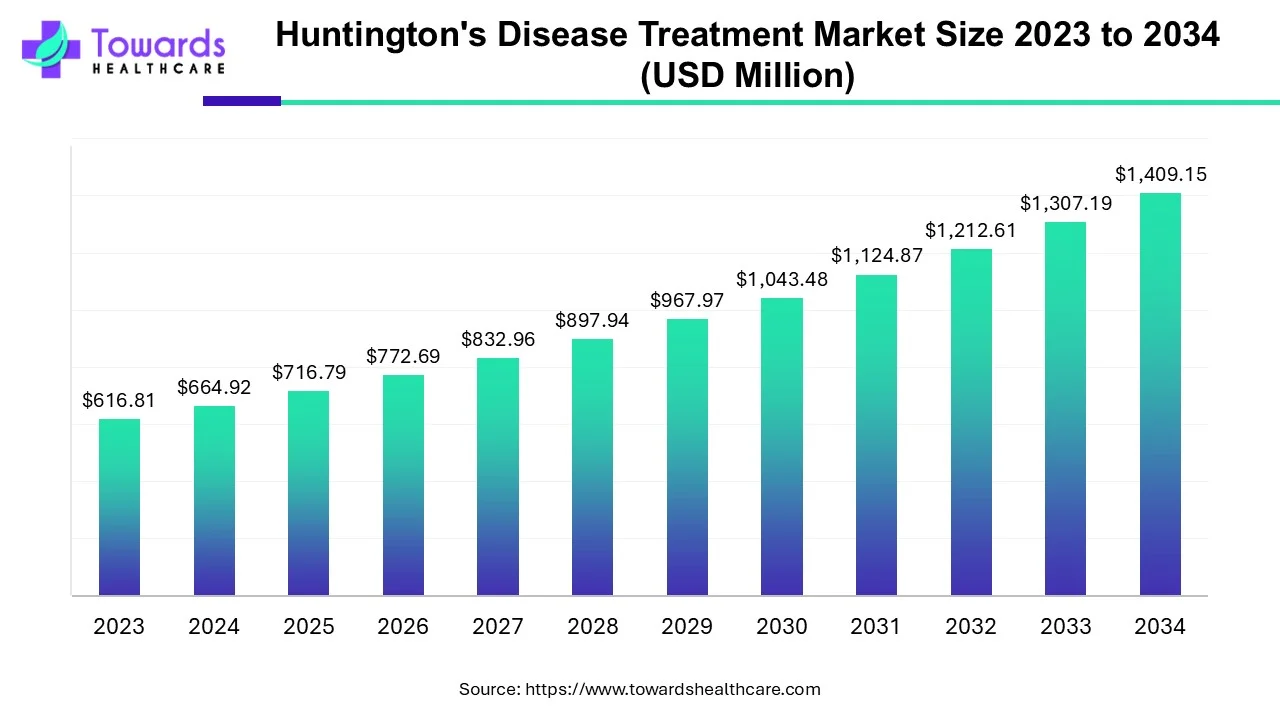
The huntington's disease treatment market is projected to reach USD 1519.02 million by 2035, growing from USD 716.79 million in 2025, at a CAGR of 7.8% during the forecast period from 2026 to 2035. The rising prevalence of Huntington’s disease, growing research and development activities, and technological advancements drive the market.
| Key Elements | Scope |
| Market Size in 2026 | USD 772.67 Million |
| Projected Market Size in 2035 | USD 1519.02 Million |
| CAGR (2026 - 2035) | 7.8% |
| Leading Region | North America |
| Market Segmentation | By Treatment, By End-Use, By Region |
| Top Key Players | Apitex Inc., Teva Pharmaceuticals Industries, Athena Diagnostic, Alnylam Pharmaceuticals, Elekta AB, Pfizer Inc., SOM BIOTECH, Armata Pharmaceuticals, Inc., H. Lundbeck A/S, Fulgent Genetics |
"Huntington’s disease affects an estimated 2.7 every 100,000 individuals The rising prevalence fosters demand for research and treatment management of huntington's disease."
Huntington's disease (HD) is an inherited condition where specific brain cells gradually break down, impacting movement control and various aspects of behavior and emotion. Individuals with huntington's disease (HD) may experience involuntary dance-like movements called chorea, which can intensify when nervous or distracted. Symptoms typically emerge in middle-aged adults but can also affect children, albeit rarely. The disease progresses, leading to increased movement, balance and cognitive function challenges.
Despite these challenges, many people with huntington's disease (HD) remain aware of their surroundings and can express emotions. Behavioral changes encompass mood swings, irritability, apathy, depression and anger, with some individuals experiencing persistent symptoms like suicidal thoughts and psychosis. Social withdrawal can occur, impacting participation in activities. It is essential to approach HD with empathy, recognizing it as a severe condition requiring comprehensive management. The global demand for effective treatments has increased as awareness of this challenging disorder grows.
Huntington's disease is a genetic disorder affecting the brain, causing movement problems, cognitive decline and emotional issues. Managing symptoms involves medications and support. Innovative solutions like gene therapies, are being explored for potential future treatments. However, it remains a complex condition to control fully and that's why scientists focus on more research and treatment.
Artificial intelligence (AI) algorithms disrupt the healthcare sector by contributing to a range of applications from research to disease treatment. AI can streamline the entire research process and assist researchers in developing novel and the most potent therapeutics for HD. It can analyze the pharmacokinetic and pharmacodynamic properties of novel molecules. AI and machine learning (ML) algorithms can screen and effectively diagnose HD. They can analyze vast amounts of patient data and categorize HD into different stages. This enables healthcare professionals to provide personalized treatment, enhancing treatment efficiency and accuracy. AI can also automate the clinical trial process by improving patient outcomes and transforming data analysis.
The global prevalence of Huntington’s disease is estimated to be 4 to 10 cases per 100,000 individuals. North America and Europe report the highest number of cases, followed by Asia and Africa.
HD is inherited in a way where having one copy of the mutated gene from either parent can lead to the disease. The expansion of a specific DNA sequence in the HD gene causes it. This gene provides instructions for making a protein called huntingtin, found throughout the body, particularly in the central nervous system. While the exact function of huntingtin isn't fully understood, it's believed to play essential roles in various cellular processes.
When the DNA sequence expands beyond the normal range, especially with paternal transmission, it can lead to HD. The threshold for developing symptoms is commonly considered when the repeats reach 36 or more, with full-blown symptoms typically seen at 40 repeats or above. More repeats are also linked to an earlier onset of the disease, a faster progression, and increased severity. Given the global prevalence and demand for management, there's an increasing need for therapeutic interventions and support for individuals affected by HD.
Huntington's disease is like a puzzle with a specific genetic piece missing or altered. The challenge lies in creating therapies that can precisely target this mutated gene responsible for HD without accidentally affecting the healthy genes around it.
Additionally, this precision work is no easy task. It's like performing delicate surgery at the molecular level. If the therapy isn't precise enough, it might unintentionally interfere with other healthy genes, potentially causing more problems than solutions. Here's where it links to the huntington's disease treatment market. The complexity of this precision challenge can slow the development of effective therapies. Research and development become more intricate, requiring extensive resources and time. This complexity can decrease the overall market activity for HD treatments as pharmaceutical companies and researchers navigate the intricate landscape of genetic intricacies. So, the difficulty in precisely targeting the root cause of HD without causing collateral damage to healthy genes creates a hurdle in developing treatments, subsequently impacting the market for huntington's disease therapies.
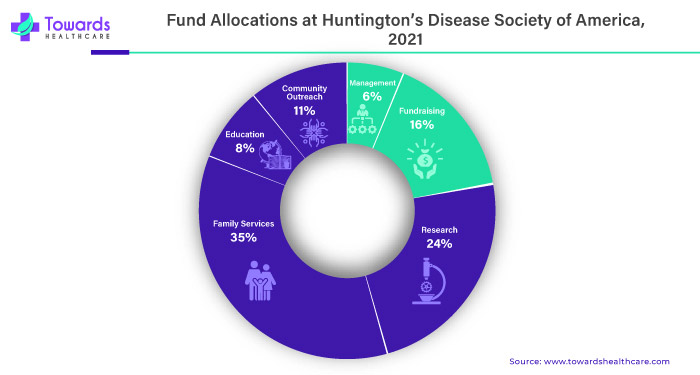
Disease-modifying therapy is like a superhero for the huntington's disease market. It's the kind of treatment that doesn't just tackle the symptoms but goes straight to the root of the problem, aiming to change the course of the disease. Imagine if we could not only ease the symptoms but also slow down or even stop huntington's disease from getting worse. That's the magic of disease-modifying therapy! It's like giving the body and brain a chance to heal and recover. If there's a therapy that can truly modify the course of the disease, it becomes a beacon of hope for those affected by huntington's. This increased hope and potential for better outcomes create a growing demand for the therapy, and where there's demand, the market naturally expands.
Pharmaceutical companies, researchers and healthcare providers also jump on board to support and develop these game-changing therapies. It's not just about managing the symptoms anymore; it's about making a real difference in the lives of those dealing with huntington's disease. So, the pursuit of disease-modifying therapy not only improves the outlook for patients but also propels the market forward with enthusiasm and optimism.
Which Treatment Segment Dominated the Huntington’s Disease Treatment Market?
By treatment, the symptomatic treatment segment held a dominant presence in the market in 2024. Symptomatic treatment refers to managing the motor, psychological, and behavioral symptoms of HD. Several pharmacological and non-pharmacological interventions are provided as part of the symptomatic treatment. Some common medications include antidepressants, antipsychotic medicines, and medicines to control movement. Technological advancements and growing research and development activities lead to the development of novel approaches for symptomatic treatment. Surgical approaches such as pallidotomy, deep brain stimulation, and fetal cell transplants have emerged as novel therapies for HD.
Disease-Modifying Therapies Segment: Fastest-Growing
By treatment, the disease-modifying therapies segment is projected to expand rapidly in the market in the coming years. Symptomatic treatment can only provide symptomatic relief, while disease-modifying treatment can target the root cause of the disease. Novel therapies are developed to alter huntingtin DNA and RNA, clear huntingtin protein, etc. The growing genomics research and increasing investments contribute to the segment’s growth. However, there are currently no U.S. FDA-approved disease-modifying treatments available for HD. Ongoing research efforts focus on developing gene therapies, oligonucleotides, and RNA interference.
Why Did the Hospital Pharmacy Segment Dominate the Huntington’s Disease Treatment Market?
By end-use, the hospital pharmacy segment will have led the global market in 2024. Hospital pharmacies have skilled professionals to evaluate prescriptions and administer the correct medications based on patients’ conditions. The increasing number of hospitalizations due to favorable reimbursement policies and specialized equipment favor hospital pharmacies. They contain a stock of all newly launched medications related to HD, providing advanced treatment care to patients.
E-commerce Segment: Significantly Growing
By end-use, the e-commerce segment is predicted to witness significant growth in the market over the forecast period. The rapidly expanding e-commerce sector and the rising adoption of smartphones boost the segment’s growth. Patients can order medications from a wide range of products at affordable prices in the comfort of their homes. The increasing geriatric population potentiates the demand for online pharmacies. Online pharmacies also offer online consultancy services, allowing patients to get state-of-the-art treatment.
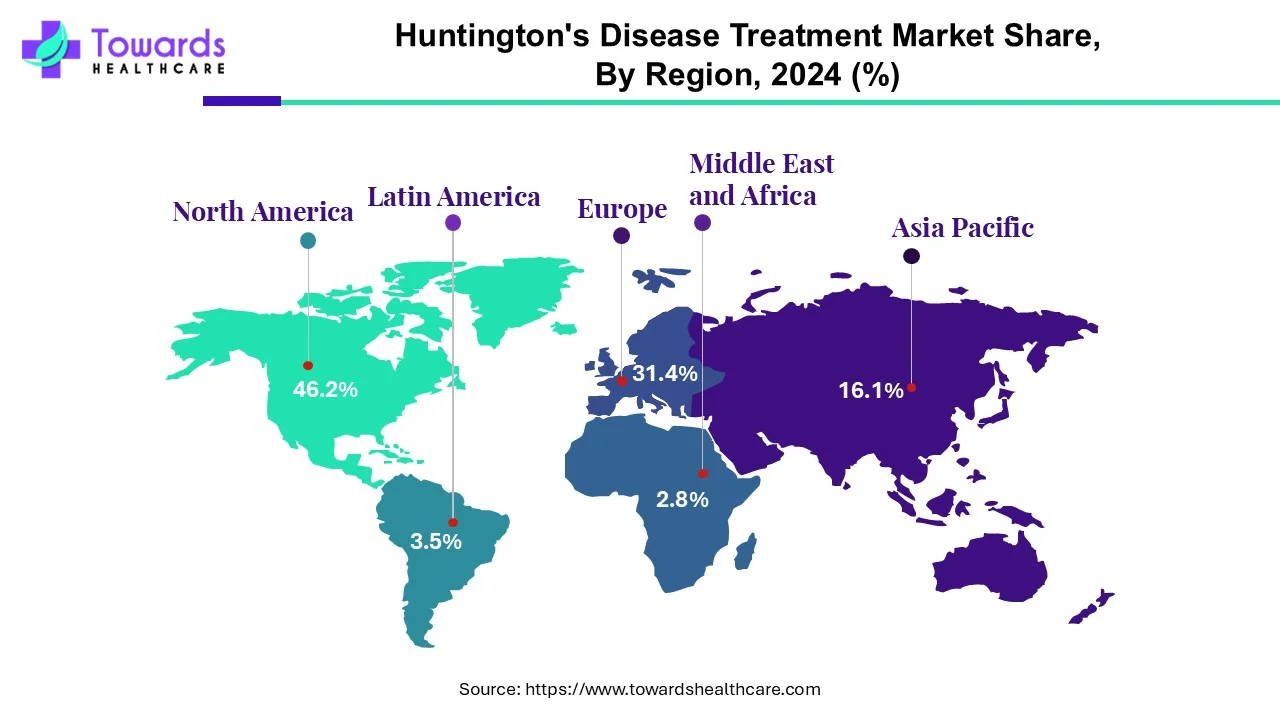
The North America region plays a significant role in the huntington's disease treatment market. Advanced healthcare infrastructure, research facilities and a higher prevalence of HD contribute to a robust market. Additionally, ongoing clinical trials and collaborations with pharmaceutical companies contribute to advancements in treatments. Favorable government policies and initiatives support the development of novel treatment regimens for HD. The National Institute of Neurological Disorders and Stroke (NINDS) provides funding to support HD research. The FY 2025 President’s Budget for NINDS was $2,833.8 million, an increase of $24.4 million from FY 2024.
Canada Market Trends
In January 2025, the Royal Philip Foundation and Brain Canada Foundation announced an investment of $600,000 each through the Canada Brain Research Fund (CBRF), totaling of $1.2 million. The funding was provided to four two-year Navigator projects in 2025 and 2026 to eradicate Huntington’s disease.
Countries like the United Kingdom, Germany, and France actively participate in HD research and development. The European Medicines Agency (EMA) regulates therapies, ensuring their safety and efficacy. Collaborations between academic institutions, pharmaceutical companies and healthcare providers drive progress in treatment options. The European Huntington’s Disease Network (EHDN) is a network dedicated to advancing research, conducting clinical trials, and improving care for HD patients. The rising prevalence of HD augments the market. According to a recent study, the prevalence of HD in Europe is estimated to be 6.37 per 100,000 individuals.
UK Market Trends
The Huntington’s Disease Association launched the “England Rare Diseases Action Plan 2024” to address health inequalities and improve the lives of people living with rare diseases across the UK. The action plan will report on progress against the existing 29 actions as well as introduce 7 new actions to improve the lives of people with rare diseases for the year ahead.
In Asia-Pacific (Japan, Korea, China), while the prevalence of Huntington's disease is relatively lower in Asian countries, the market is evolving. Increased awareness, growing healthcare infrastructure and rising research initiatives contribute to expanding the HD treatment market in this region. The rising geriatric population and favorable government support also contribute to market growth. Countries like China, India, and Japan are at the forefront of developing novel treatments for HD. Several government organizations have launched initiatives for screening and early detection of HD.
China Market Trends
According to a recent nationwide cross-sectional study published in Neuroepidemiology, 80.6% of 269 individuals with HD were receiving treatment. The average annual direct medical cost, direct non-medical cost, and indirect cost were 3,265.65, 805.82, and 801.97 Euros, respectively.
Japan Market Trends
The Japanese government conducted a large-scale research project to digitally reproduce human brain functions to tackle cranial nerve-related diseases, such as dementia and depression. It is estimated that nearly 10% of people in Japan are living with cognitive decline and is projected to reach 5.93 million by 2030.
The Middle East & Africa are expected to grow at a notable CAGR in the foreseeable future. The rising prevalence of HD, advances in diagnostic technologies, and the growing demand for personalized therapeutics bolster market growth. The burgeoning healthcare sector and growing research activities foster the market, improving treatment outcomes. The rising adoption of advanced technologies and the increasing R&D investments also contribute to market growth.
UAE Market Trends
Healthcare organizations, such as Emirates Hospitals and German Neuroscience Center Dubai, provide state-of-the-art treatment to HD patients. The mean prevalence of HD in Arab countries is 1.74 cases per 100,000 inhabitants. The UAE government has launched a roadmap and an action plan to address the growing burden of neurological diseases in the region.
Latin America is expected to grow at a considerable CAGR in the upcoming period. People are becoming aware of early diagnosis of neurological disorders, enabling healthcare professionals to provide early intervention. Government organizations launch initiatives and organize screening campaigns for HD patients. They also promote newborn screening to detect the risk of HD, owing to genetic predispositions.
Brazil Market Trends
The Brazilian government makes constant efforts to achieve a uniform coverage of its neonatal screening programs for the early detection and intervention to improve health outcomes. The growing geriatric population poses a greater risk of developing neurological disorders. As of 2025, there were over 31 million people aged 60 years and above.
Alicia Secor, CEO and President of Atalanta Therapeutics, commented on raising $97 million in a Series B funding that this financing validates the transformative potential of the di-siRNA platform for delivering oligonucleotide therapies to the CNS. The funding will help develop therapeutics for KCNTI-related epilepsy and Huntington’s disease (HD) and would anchor the growing franchise of investigational medicines for HD.

By Treatment
By End-Use
By Region