The Brands Driving Advances in Clinical Trials Market
- Parexel
- IQVIA
- Charles River Laboratory
- Omnicare
- Kendle
- Chiltern
- Pharmaceutical Product Development, LLC
Clinical Trials Market Overview
R&D spending on pharmaceutical companies helps in the innovation of new drug development-boosting clinical trials market.
A clinical trial is a collection and integration validation of clinical trial data. Clinical trials can be divided into five phases, with every phase playing a distinct purpose within the clinical trial. Every trial adheres to a procedure that designates what types of individuals may participate in the study. Clinical trials are conducted for many reasons to establish the effectiveness and safety of a new medicine or device for human use and research other approaches of using current, approved treatments or standard treatments to make them more usable, more effective, or have fewer negative effects, gain knowledge about how to safely provide treatment to a group for which it has not yet undergone testing, such as youngsters.
The trials also outline the exact plan of procedures, tests, medications, and doses within the trial apart from specifying the span of the study. In recent years, the costs associated with drug development have increased significantly, driving pharma and biotech companies to look for modernizations and smarter ways of conducting business. One important trend is the outsourcing of clinical research activities by manufacturers. By subcontracting their R&D activities, pharma and biotech companies are reforming the drug development facilities business. The R&D service providers have risen from just a few establishments providing restricted clinical trial facilities to big conglomerates offering an extensive range of facilities like study design, preclinical evaluations, clinical trial management and planning, autonomous safety data audit, bio-statistical analysis, and several more.
Market Growth
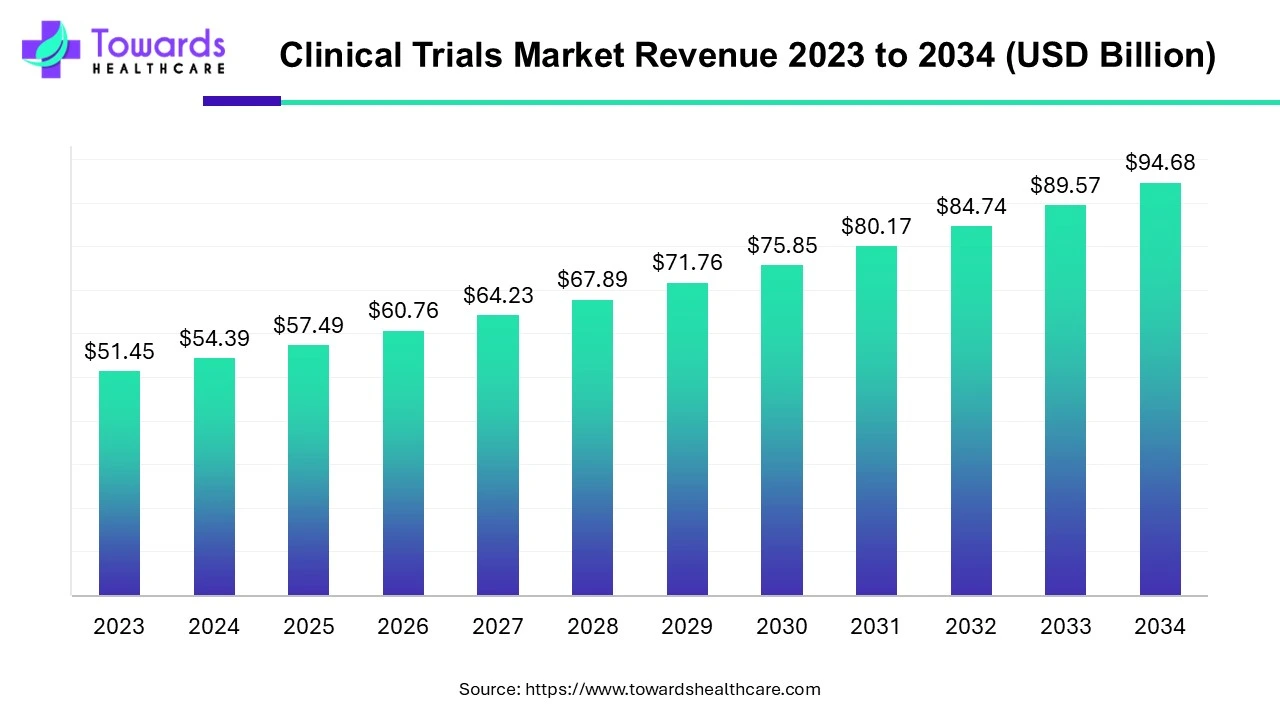
The global clinical trials market size is calculated at USD 54.39 billion in 2024 and is expected to be worth USD 94.68 billion by 2034, expanding at a CAGR of 5.7% from 2024 to 2034.
Clinical Trials Market Trends
- Growing R&D: The increasing incidences and prevalence of several chronic disorders and research on various topics, such as novel drug discovery & development, personalized medicines, biologics, cell and gene therapy, medical devices, etc., drive the market.
- Increasing Investments: Several government and private organizations fund clinical trial projects and collaborate to foster growth in the field.
- Favorable Government Policies: Government policies and initiatives to support clinical trials and develop scientific infrastructure.
- Technological Advancements: Advanced technologies streamline the entire clinical trial process, providing faster and more accurate results.
Latest Announcements by Industry Leaders
- Badhri Srinivasan, Head of Global Clinical Operations, Novartis stated that the Indian regulators are modifying regulations to make clinical trials easier, more accelerated, more accessible, etc. India will have more transparency, standardization, and clarity on what the ethics committee can do.
- In January 2025, Tom O’Leary, CIO at ICON plc announced the expansion of its portfolio of AI tools that deliver efficiencies across the clinical trial process, including study startup, document management, resource forecasting, and metrics reporting for developing and deploying AI solutions that hasten trials, improve data, and optimise operational efficiencies.
- In April 2024, Jonathan Shough, Chief Information Officer for Parexel, announced a multi-year strategic partnership between Parexel and Palantir Technologies Inc. to exploit AI to help improve and hasten the delivery of safe and effective clinical trials for the biopharmaceutical customers of the world. Thus, the partnership emphasized driving clinical trial efficiency as well as maintaining the highest level of safety and regulatory rigor.
Recent Developments
- In June 2024, IQVIA Digital Products and Solutions announced the launch of One Home for Sites™, as a new platform to streamline clinical trial management for research sites because it acts as a single sign-on and a single dashboard for the key systems and tasks across all the clinical trials conducting on one platform by freeing up time and conducting more research with patient care.
- In October 2024, with a maximum expenditure of £400 million, the UK government announced the start of the Voluntary Scheme for Branded Medicine Pricing, Access and Growth (VPAG) investment initiative, which aims to improve clinical trials.
- In January 2023, Paradigm opened a $203 million fundraising round to increase access to clinical trials. The money will be used to expand the company's platform and build alliances with healthcare systems and life science businesses.
Collaborate with our experts to explore the Clinical Trials Market at sales@towardshealthcare.com
Keypoints
- Company Overview
- Locations Subsidiaries/Geographic reach
- Key Executives
- Company Financials
- Patents registered
- SWOT Analysis
- Applications Catered
- Strategic collaborations
- Recent Developments
- Competitive Benchmarking
