Revenue, 2025
USD 92.88 Billion
Forecast, 2035
USD 174.18 Billion
Clinical Trials Market Size, Growth Analysis and Latest Trends
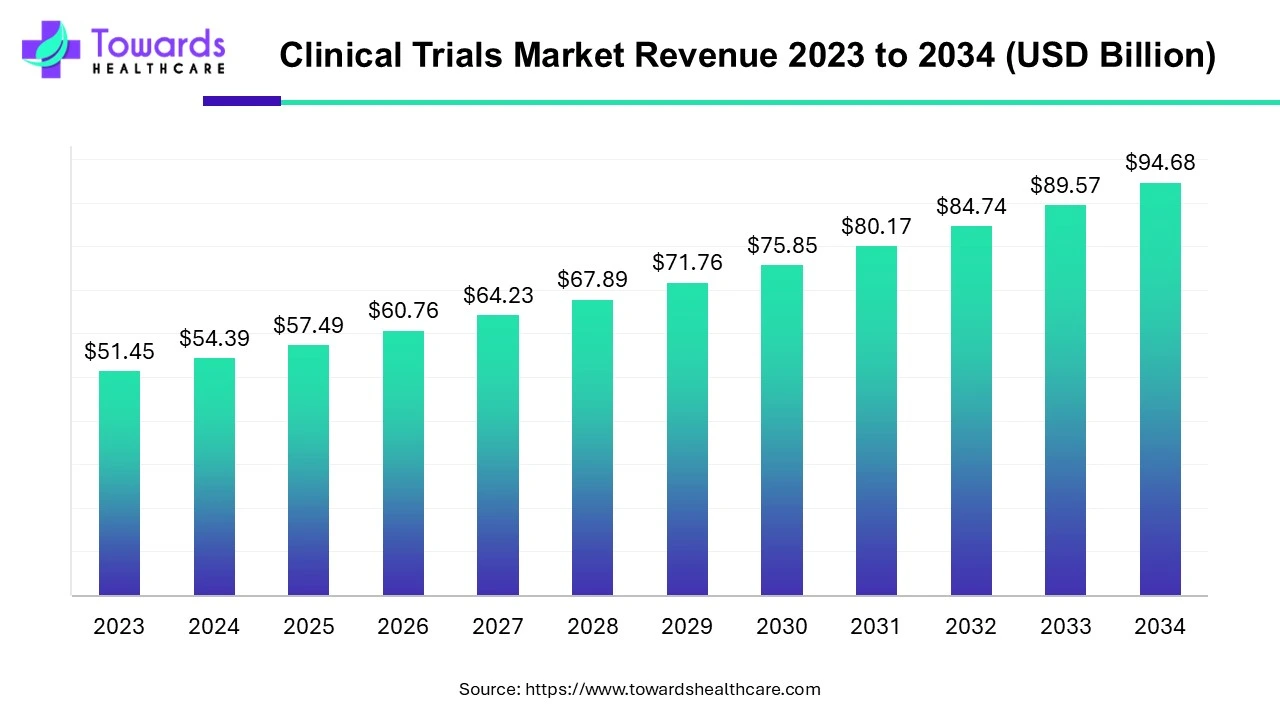
The global clinical trials market size is calculated at USD 98.91 billion in 2026 and is expected to be worth USD 174.18 billion by 2035, expanding at a CAGR of 5.7% from 2026 to 2035.
Key Takeaways
- Clinical trials sector pushed the market to USD 92.88 billion by 2025.
- Long-term projections show USD 174.18 billion valuation by 2035.
- Growth is expected at a steady CAGR of 5.7% in between 2026 to 2035.
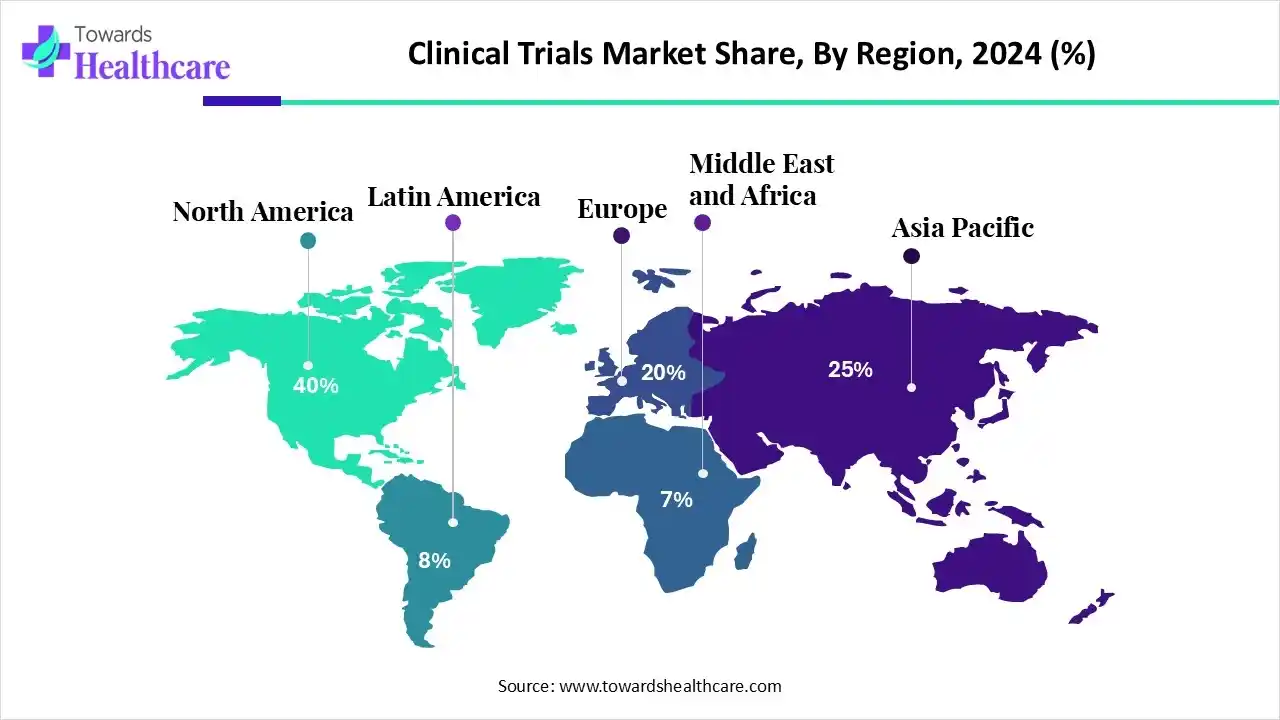
- North America dominated the clinical trials market by 60% in 2024.
- Asia Pacific is estimated to be the fastest-growing during the forecast period.
- By phase, the phase 3 segment led the market in 2024.
- The North America clinical trials market is set to grow from US$ 22.52 billion in 2025 to US$ 30.69 billion by 2034 at a CAGR of 3.50%.
- The Middle East and Africa clinical trials market, valued at US$ 3.81 billion in 2024, is projected to reach US$ 7.36 billion by 2034, growing at a CAGR of 6.80%.
- The Europe clinical trials market is projected to grow from US$ 14.25 billion in 2025 to US$ 21.73 billion by 2034 at a 4.8% CAGR.
- The Latin America clinical trials market is projected to grow from US$ 4.35 billion in 2024 to US$ 7.94 billion by 2034 at a 6.2% CAGR.
- The APAC clinical trials market is expected to grow from US$ 10.88 billion in 2024 to US$ 26.96 billion by 2034 at a 9.5% CAGR.
- By phase, the phase 2 segment is anticipated to grow at the fastest rate during the predicted time.
- By study design, the interventional study segment dominated the market in 2024 and is estimated to grow at the fastest rate during the forecast period.
- By intervention, the oncology segment led the market in 2024 and is estimated to achieve the fastest CAGR during the forecast period.
Key Indicators and Highlights
| Key Elements |
Scope |
| Market Size in 2025 |
USD 92.88 billion |
| Projected Market Size in 2035 |
USD 174.18 billion |
| CAGR (2025 - 2035) |
5.7% |
| Leading Region |
North America by 60% |
| Market Segmentation |
By Phase, By Study Design, By Indication, By Geography |
| Top Key Players |
Parexel, IQVIA, Charles River Laboratory, Omnicare, Kendle, Chiltern, Pharmaceutical Product Development, LLC |
Clinical Trials Market Trends
- Growing R&D: The increasing incidences and prevalence of several chronic disorders and research on various topics, such as novel drug discovery & development, personalized medicines, biologics, cell and gene therapy, medical devices, etc., drive the market.
- Increasing Investments: Several government and private organizations fund clinical trial projects and collaborate to foster growth in the field.
- Favorable Government Policies: Government policies and initiatives to support clinical trials and develop scientific infrastructure.
- Technological Advancements: Advanced technologies streamline the entire clinical trial process, providing faster and more accurate results.
Clinical Trials Market Overview
R&D spending on pharmaceutical companies helps in the innovation of new drug development-boosting clinical trials market.
A clinical trial is a collection and integration validation of clinical trial data. Clinical trials can be divided into five phases, with every phase playing a distinct purpose within the clinical trial. Every trial adheres to a procedure that designates what types of individuals may participate in the study. Clinical trials are conducted for many reasons to establish the effectiveness and safety of a new medicine or device for human use and research other approaches of using current, approved treatments or standard treatments to make them more usable, more effective, or have fewer negative effects, gain knowledge about how to safely provide treatment to a group for which it has not yet undergone testing, such as youngsters.
The trials also outline the exact plan of procedures, tests, medications, and doses within the trial apart from specifying the span of the study. In recent years, the costs associated with drug development have increased significantly, driving pharma and biotech companies to look for modernizations and smarter ways of conducting business. One important trend is the outsourcing of clinical research activities by manufacturers. By subcontracting their R&D activities, pharma and biotech companies are reforming the drug development facilities business. The R&D service providers have risen from just a few establishments providing restricted clinical trial facilities to big conglomerates offering an extensive range of facilities like study design, preclinical evaluations, clinical trial management and planning, autonomous safety data audit, bio-statistical analysis, and several more.
Market Dynamics
Patient Recruitment and Regulatory Challenges Hinder the Clinical Trials Market
The major challenge of the market is the patient recruitment for clinical trials. Clinical trials require both healthy and diseased patients to assess the safety and efficacy of novel medicinal products. However, the lack of sufficient safety data and risk of adverse effects restricts the participation of subjects in clinical trials, thereby hindering the market.
Another major challenge is the stringent regulatory guidelines. Organizations need to follow the regulations of every country in which the clinical trial is conducted. This makes the process more complex and hampers the market.
An Increase in Investment in Pharmaceutical Research & Development Drives Market Growth
Most healthcare, pharmaceutical, biopharmaceutical, and medical device companies continue working on research and development and heavily invest in the development of novel drugs and devices. Pharmaceutical companies highly invest in research and development to deliver high-quality and innovative products for patients. Nowadays top pharma companies are increasing their research and development efficiencies through high investment in R&D and collaborative efforts. The increase in research and development expenditure of pharmaceutical and biopharmaceutical companies is prompting them to opt for providing fully integrated or functional outsourcing services, from the early development phase to the last phase for both drug development and discovery.
Big pharmaceutical companies have enormous pressure for a fixed cost of the drugs and time limit for development as they fully involve in outsourcing drugs, most pharmaceutical and biopharmaceutical companies tend to outsource their testing functions during R&D to improve profitability, to meet the timelines involved in drug development, and save costs. The increasing number of drugs required outsourcing various drug development stages to manage capacities and access scientific and process innovations to develop cost-effective and efficient drug molecules.
This trend is expected to drive the growth of the clinical trial market. The industry is expected to grow as technologically sophisticated digital solutions become more widely used with several government initiatives. Another factor driving the burden of chronic disease demand for clinical trials is the growing elderly population. With the rising prevalence of chronic diseases, pharmaceutical companies have increased their attention to creating novel treatments for rare or hereditary diseases that necessitate specialized clinical studies. This is expected to accelerate the growth of the sector.
Crucial factors accountable for market growth are:
- Growing prevalence of chronic disorders
- Increasing number of clinical trials in developing regions
- Growing number of biologics
- Increasing demand for advanced treatments such as personalized medicines
A Growing number of Clinical Trials Outsourcing services to CROs Future the Market Growth
The increased uptake of cutting-edge medical technologies and the expanding demand for novel pharmaceuticals are driving up demand for the clinical trials sector. Additionally, the high costs and substantially lower approval rates of the medication development process make it exceedingly dangerous for pharmaceutical and biotechnology companies, which restricts the market growth for clinical trials. As a result, contract research organizations (CROs) are increasingly being used to conduct clinical trials, which allows sponsors to focus primarily on the development of new drugs and improves the prognosis for the market. Additionally, the size and number of clinical trials have increased as the commercialization of clinical research spreads from Europe and the US to most of the world. As a result, pharmaceutical companies are under more pressure to meet deadlines. Even huge pharmaceutical companies are finding it challenging to handle the rising workload, which is driving up outsourcing. So, during the projection period, the market is expected to grow as demand for clinical trial outsourcing rises.
Segmentation Analysis
Study Design Insights
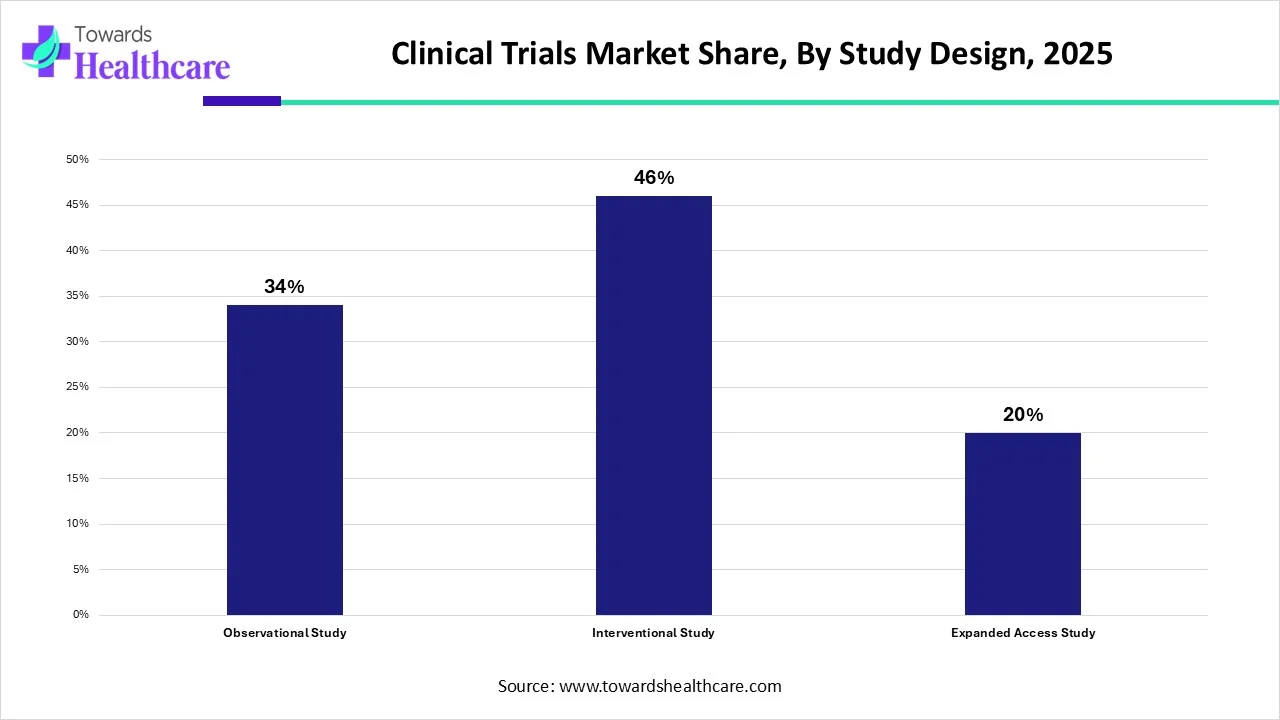
Which Study Design Segment Dominated the Clinical Trials Market?
The requirement for clinical trials in developing diagnostic tests and vaccines for viral diseases such as SARS-CoV-2 has augmented the demand for clinical trials exponentially. Thus, the high incidence of novel viral diseases and ongoing technological improvements in clinical trials are major reasons for the high revenue share of interventional studies. The interventional study segment is expected to grow at a CAGR of 5.4% from 2022 to 2030. The interventional design segment accounted largest market share 46% in 2025.
Indication Insights
Why Did the Oncology Segment Dominate the Clinical Trials Market?
Constant research on cancer treatment and increasing demand for precision medicine are the major reasons responsible for the high market share of oncology. The oncology segment was valued at USD 25 billion by 2030. The oncology segment accounted for a revenue share of 24% in 2021 and is expected to witness growth at a CAGR of 6.4% over the forecast period from 2022 to 2030.
Regional Insights
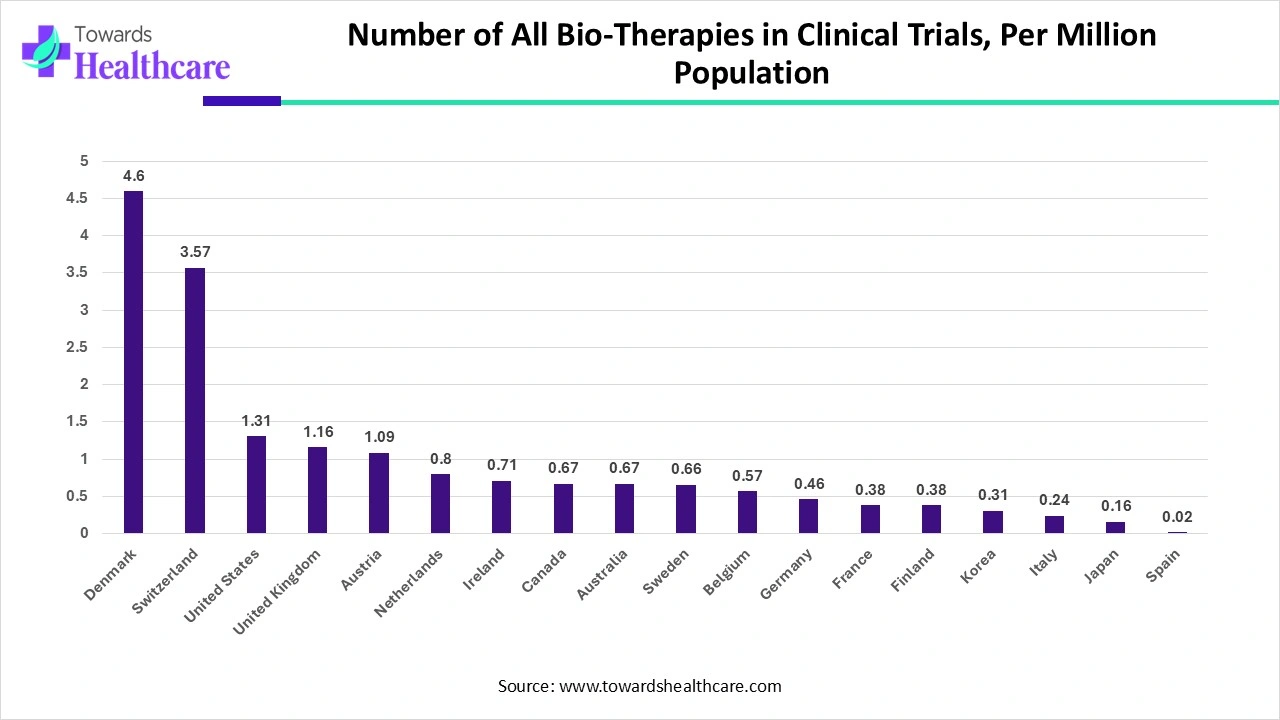
Number of Bio-Therapies and Experimental Bio-Therapies in Clinical Trials, December 2007
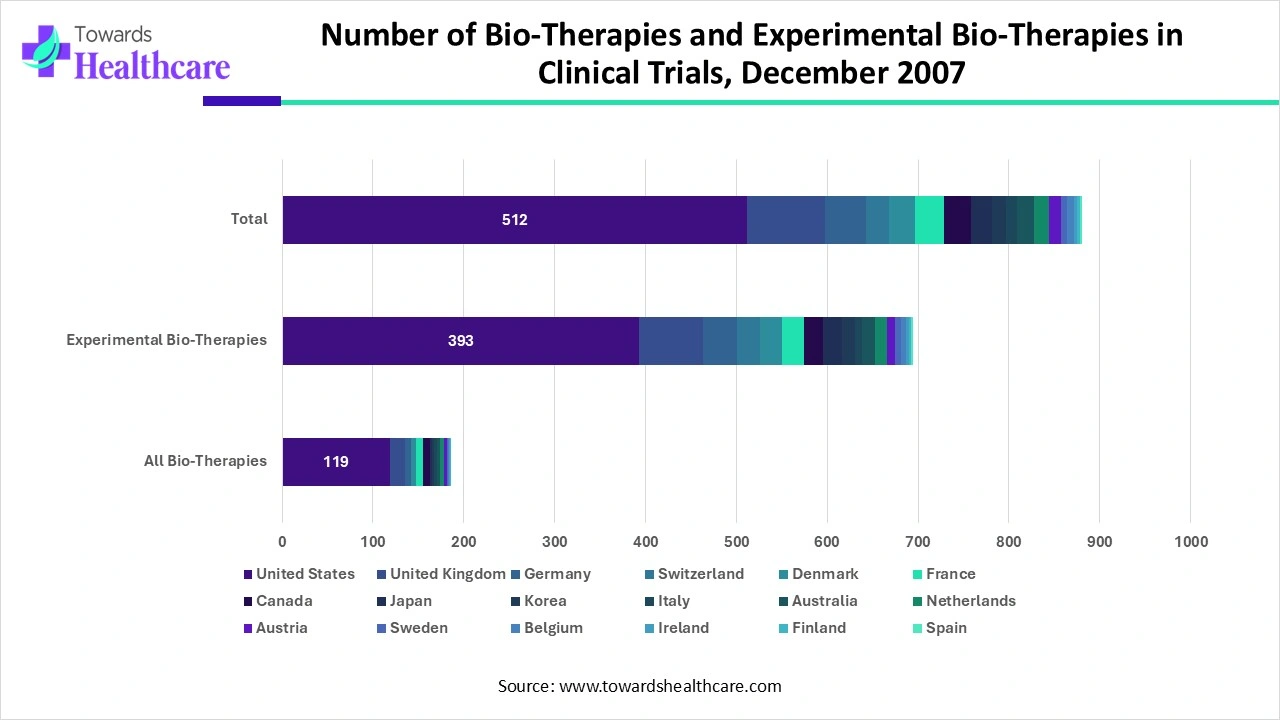
Increasing Investments Dominated North America
The North America clinical trials market is valued at US$ 55.48 billion in 2025, expected to grow to US$ 58.82 billion in 2026, and projected to reach approximately US$ 99.10 billion by 2035. This reflects a compound annual growth rate (CAGR) of 5.40% from 2026 to 2035.
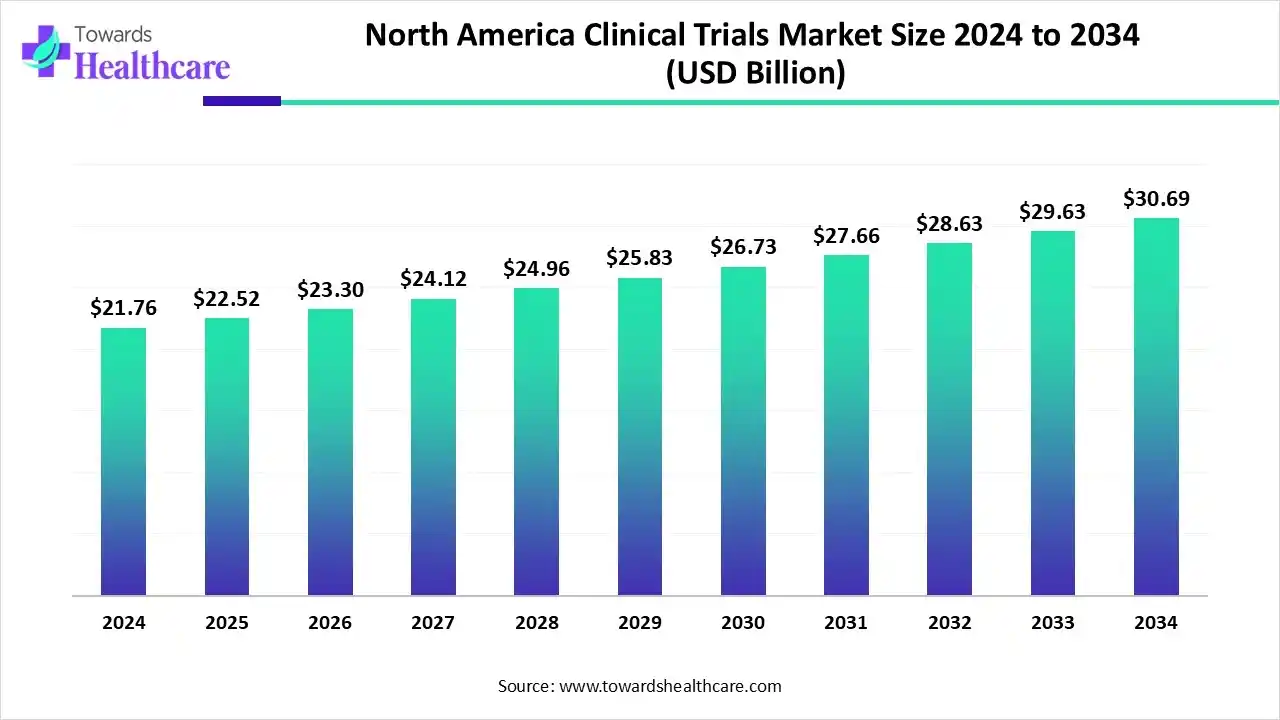
North America held the largest share of the clinical trials market in 2023. The increasing number of clinical trials, the presence of major pharmaceutical & biotechnology giants, technological advancements, and favorable scientific infrastructure drive the market. Clinical trials in the US are governed by the Food and Drug Administration. The US FDA ensures that clinical trials are designed, conducted, analyzed, and reported according to the laws. Health Canada is responsible for the conduct of clinical trials in Canada. The National Institute of Health invests most of its $48 billion budget in medical research for Americans. In August 2023, Project NextGen by the US Department of Health and Human Services granted $1 billion for COVID-19 vaccine clinical trials and $326 million for monoclonal antibodies.
Rising Number of Clinical Trials Drives Asia-Pacific
The APAC clinical trials market size was estimated at US$ 18.87 billion in 2025, projected to increase to US$ 20.42 billion in 2026 and reach US$ 41.33 billion by 2035, showing a healthy CAGR of 7.16% across the forecast years.
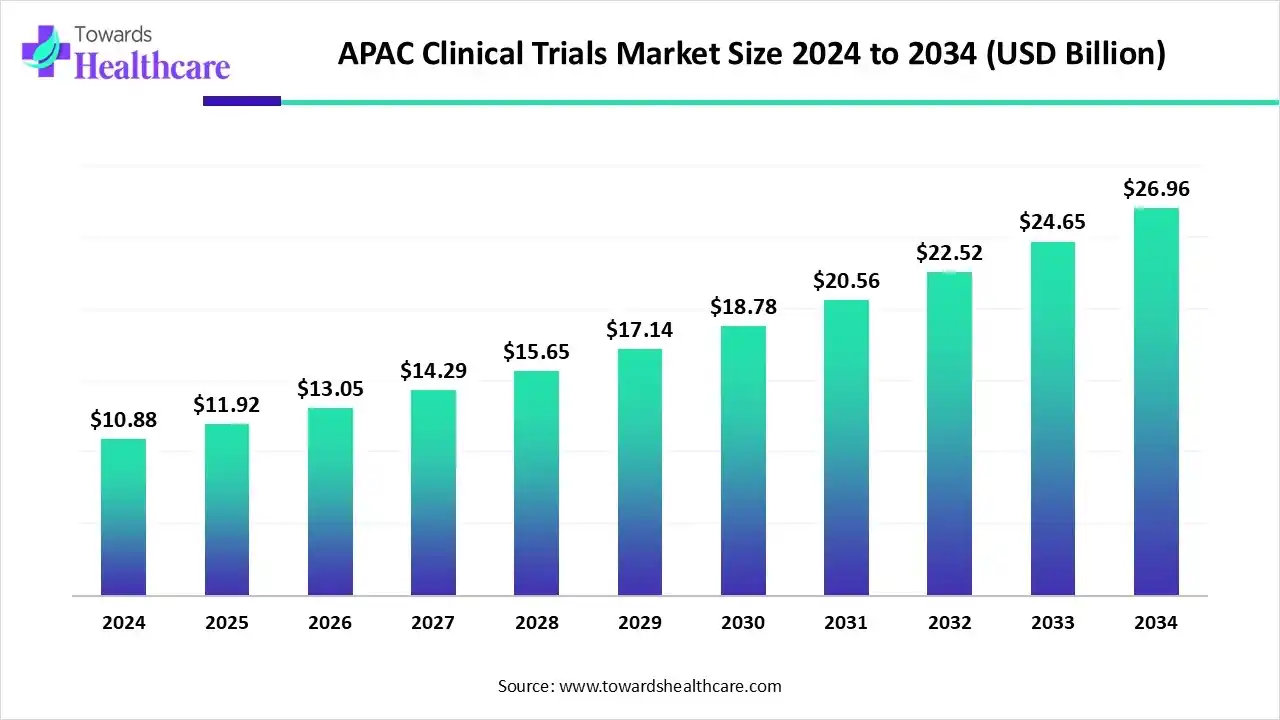
Asia-Pacific is anticipated to grow at the fastest rate in the market during the forecast period. The rising incidences of chronic disorders, expanding healthcare infrastructure, increasing number of clinical trials, and increasing investments & collaborations drive the market. Clinical trials are rapidly increasing in developing countries like India, Thailand, Vietnam, Philippines, etc. India reports a surge in phase 2 and 3 clinical trials from 2017 to 2023, growing from 15% to 18%. Similarly, in China, 4,300 clinical trial registrations were done in 2023, accounting for a 26.1% surge from 2022. China also reports an average consistent 16% growth in five-year clinical trials.
Oncology and Rare Diseases in Europe: Driving the Market
The Europe clinical trials market is valued at US$ 11.04 billion in 2025, increasing to US$ 11.65 billion in 2026, and is expected to reach approximately US$ 18.81 billion by 2035. This growth reflects a CAGR of 4.80% during the forecast period from 2026 to 2035.
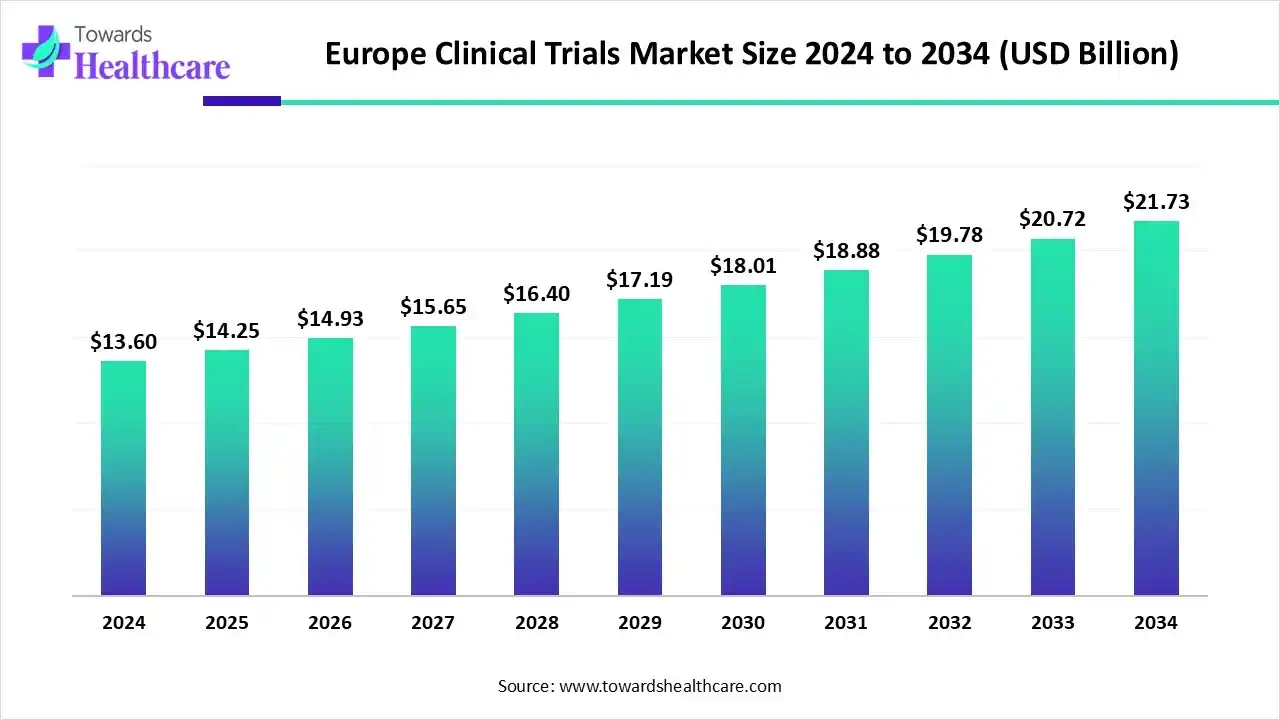
Europe is expected to grow at a notable rate in the foreseeable future in the clinical trials market because of its high-quality healthcare systems, diverse patient populations, and a strong regulatory framework. Europe, by offering access to specialized medical professionals, robust infrastructure, and systematic patient recruitment, facilitates efficient data collection. Oncology and rare diseases are conspicuously noticeable therapeutic areas for clinical trials in Europe, together with Central and Eastern Europe is rising as a preferred place for clinical trials owing to its increased and diverse patient populations, minimal costs, and clear regulatory frameworks. Furthermore, Europe is a major hub for both public and private funding of clinical research, contributing to its prominence in the global market.
The UK is becoming a global hub for clinical trials, accounting for a total of 27,643 trials as of December 2025. The UK government recently announced an investment of £400 million to support faster patient access to cutting-edge treatments, strengthen clinical trials, and improve medicines manufacturing in the UK. 18 new clinical trial hubs will be created across the UK to accelerate research as part of this funding.
Latin America Clinical Trials: PAHO Drives Growth, Ethics, and Global Collaboration
The Latin America clinical trials market is valued at US$ 4.67 billion in 2025 and is expected to grow to US$ 5.0 billion in 2026. It is projected to reach approximately US$ 9.17 billion by 2035, expanding at a compound annual growth rate (CAGR) of 6.30% from 2026 to 2035.
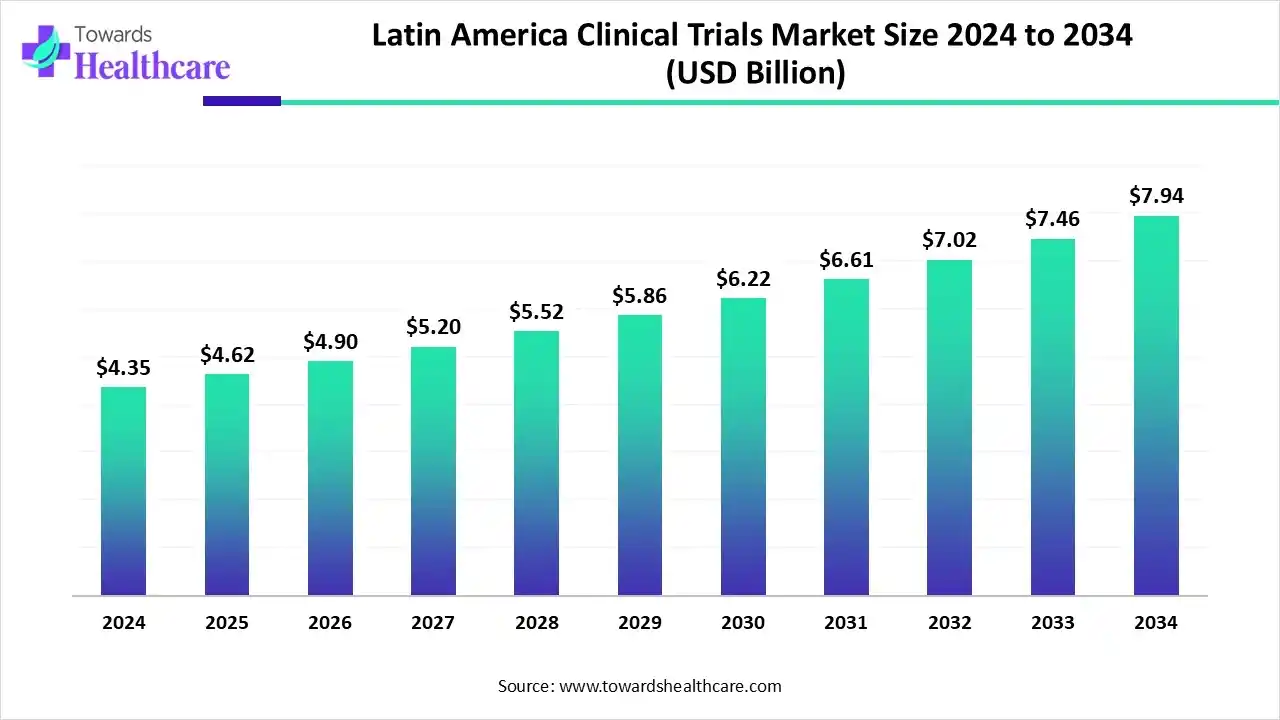
The Pan American Health Organization (PAHO) made efforts to expand the Latin America clinical trials market, improve clinical trials, offer high-quality results on health interventions, and improve research quality and coordination. The establishment of networks and collaboration supports the conduct of high-impact clinical trials and the expansion of research capacities. Advancing ethical and regulatory efficiency is of utmost importance to review and assess trials for their safety and effectiveness. The International Clinical Trials Registry Platform (ICTRP) of the World Health Organization (WHO) is a public platform that provides the required information on clinical trials conducted globally.
Brazil’s population diversity plays a key role in generating representative data of broader populations. Thus, the Brazilian regulatory agency has established the first centralized and a more structured regulatory framework to regulate human subject research in Brazil. As of December 2025, 10,996 studies were registered in Brazil on the clinicaltrials.gov website.
Middle East and Africa Clinical Trials Market Driven by Rising Chronic Diseases and Research Investments
The Middle East and Africa clinical trials market was valued at US$ 2.81 billion in 2025 and is expected to reach US$ 3.03 billion in 2026, progressing steadily to approximately US$ 5.79 billion by 2035. This growth reflects a compound annual growth rate (CAGR) of 6.80% over the forecast period.
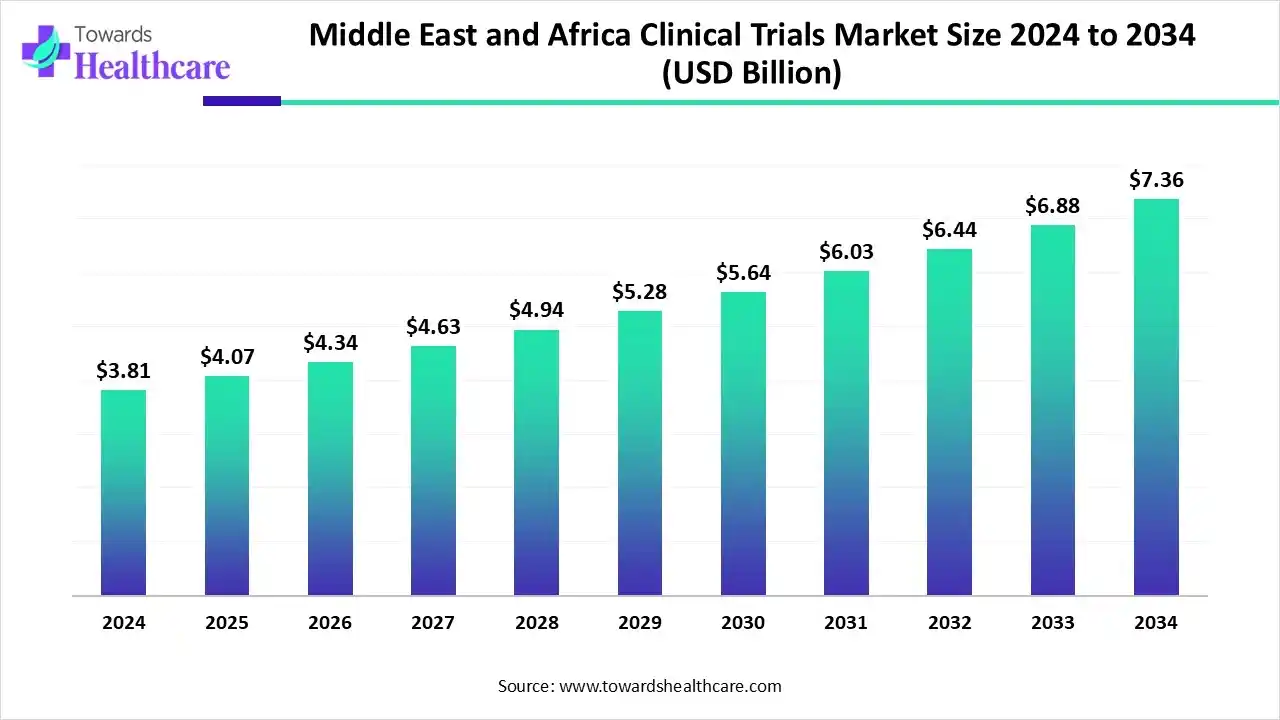
The Middle East and Africa clinical trials market is witnessing substantial growth, fueled by the increasing incidence of chronic and lifestyle-related diseases, expanding healthcare infrastructure, and favorable government policies. The region has become an attractive hub for global pharmaceutical and biotechnology companies, particularly due to South Africa’s strong presence offering advanced medical facilities, skilled clinical research professionals, and a diverse patient pool.
The UAE government is making constant efforts to establish a suitable research and clinical trial infrastructure in the region. The growing demand for personalized medicines and innovative biologics encourages local companies and CROs to conduct clinical trials to assess their safety and efficacy. Foreign companies also conduct clinical trials in the region to expand their product pipeline in the region.
Latest Announcements by Industry Leaders
- Badhri Srinivasan, Head of Global Clinical Operations, Novartis stated that the Indian regulators are modifying regulations to make clinical trials easier, more accelerated, more accessible, etc. India will have more transparency, standardization, and clarity on what the ethics committee can do.
- In January 2025, Tom O’Leary, CIO at ICON plc announced the expansion of its portfolio of AI tools that deliver efficiencies across the clinical trial process, including study startup, document management, resource forecasting, and metrics reporting for developing and deploying AI solutions that hasten trials, improve data, and optimise operational efficiencies.
- In April 2024, Jonathan Shough, Chief Information Officer for Parexel, announced a multi-year strategic partnership between Parexel and Palantir Technologies Inc. to exploit AI to help improve and hasten the delivery of safe and effective clinical trials for the biopharmaceutical customers of the world. Thus, the partnership emphasized driving clinical trial efficiency as well as maintaining the highest level of safety and regulatory rigor.
Collaboration Landscape: Clinical Trials Market
- In December 2025, Sri Sathya Sai Institute of Higher Medical Sciences (SSSIHMS) collaborated with Siemens Healthineers to combine SSSIHMS’ free medical care and Siemens Healthineers’ leadership of cutting-edge ultrasound and cardiovascular imaging.
- In December 2025, the University of Chicago Medicine announced an extended collaboration agreement with AbbVie through 2027 to support innovative solutions in cancer research and clinical trials.
- In November 2025, Biogen, Inc. and Dayra Therapeutics announced a collaboration to discover and develop oral macrocyclic peptides for priority targets in immunological conditions.
Recent Developments
- In June 2024, IQVIA Digital Products and Solutions announced the launch of One Home for Sites™, as a new platform to streamline clinical trial management for research sites because it acts as a single sign-on and a single dashboard for the key systems and tasks across all the clinical trials conducting on one platform by freeing up time and conducting more research with patient care.
- In October 2024, with a maximum expenditure of £400 million, the UK government announced the start of the Voluntary Scheme for Branded Medicine Pricing, Access and Growth (VPAG) investment initiative, which aims to improve clinical trials.
- In January 2023, Paradigm opened a $203 million fundraising round to increase access to clinical trials. The money will be used to expand the company's platform and build alliances with healthcare systems and life science businesses.
Clinical Trials Market Companies
- Parexel
- IQVIA
- Charles River Laboratory
- Omnicare
- Kendle
- Chiltern
- Pharmaceutical Product Development, LLC
Explore further to see how top key players are transforming the Clinical Trials Market at: https://www.towardshealthcare.com/companies/clinical-trials-companies
Major Market Segments Covered
By Phase
- Phase 1
- Phase 2
- Phase 3
- Phase 4
By Study Design
- Observational Study
- Interventional Study
- Expanded Access Study
By Indication
- Autoimmune/Inflammation
- Rheumatoid arthritis
- Multiple Sclerosis
- Osteoarthritis
- Irritable Bowel Syndrome (IBS)
- Others
- Pain Management
- Chronic Pain
- Acute Pain
- Oncology
- Blood Cancer
- Solid Tumors
- Other
- CNS Condition
- Epilepsy
- Parkinson's Disease (PD)
- Huntington's Disease
- Stroke
- Traumatic Brain Injury (TBI)
- Amyotrophic Lateral Sclerosis (ALS)
- Muscle Regeneration
- Others
- Diabetes
- Obesity
- Cardiovascular
- Others
By Geography
- North America
- Germany
- France
- United Kingdom
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Rest of Latin America
- Middle East & Africa (MEA)
- GCC
- North Africa
- South Africa
- Rest of Middle East & Africa