Latest Updates of Key Players in the Defibrillator Market
Medtronic
Headquarter: United States
Latest Update: In April 2025, Medtronic plc, a global leader in healthcare technology, received U.S. Food and Drug Administration (FDA) approval for the OmniaSecure defibrillation lead for placement within the right ventricle.
Abbott
Headquarter: United States
Latest Update: In January 2025, Abbott entered into a strategic collaboration with AtaCor Medical to develop an investigational extravascular implantable cardioverter defibrillator (EV-ICD) system, with the goal to enhance the safety and effectiveness of therapy for patients at risk of sudden cardiac death.
Boston Scientific Corporation
Headquarter: United States
Latest Update: In July 2025, Boston Scientific Corporation (BSC) began informing clinicians of the potential for significantly increasing low-voltage shock impedance (LVSI) associated with shock coil calcification in RELIANCE™ defibrillation leads coated with expanded polytetrafluoroethylene.
BIOTRONIK SE & Co. KG
Headquarter: Germany
Latest Update: Recent research suggested shorter-than-expected battery life for BIOTRONIK tools, prompting a significant internal analysis.
MicroPort Scientific Corporation
Headquarter: China
Latest Update: In December 2025, MicroPort Scientific Corporation announced that the strategic merger between its subsidiaries MicroPort CardioFlow Medtech Corporation and MicroPort Cardiac Rhythm Management had been approved by MicroPort CardioFlow’s shareholders.
Koninklijke Philips N.V.
Headquarter: Netherlands
Latest Update: In January 2025, Royal Philips, a worldwide leader in health technology, announced that it had signed an agreement to sell its Emergency Care business.
Value Chain Analysis – Defibrillator Market
R&D:
- Research and development for defibrillators is a multi-stage process that combines biomedical engineering, electrical design, and rigorous clinical validation to safeguard life-saving reliability.
- Key Players: Medtronic and Abbott
Manufacturing Processes:
- Defibrillator manufacturing includes an integration of precision electronics assembly for the device itself and, particularly, sterile, material-science-based technology for the electrode pads.
- Key Players: Boston Scientific and Stryker
Patient Services:
- Patient services involved in defibrillators involves wide range of care, including preliminary tests, surgical implantation, and long-term monitoring, often with help from a dedicated medical care team.
- Key Players: ZOLL Medical and Biotronik
Market Forecast
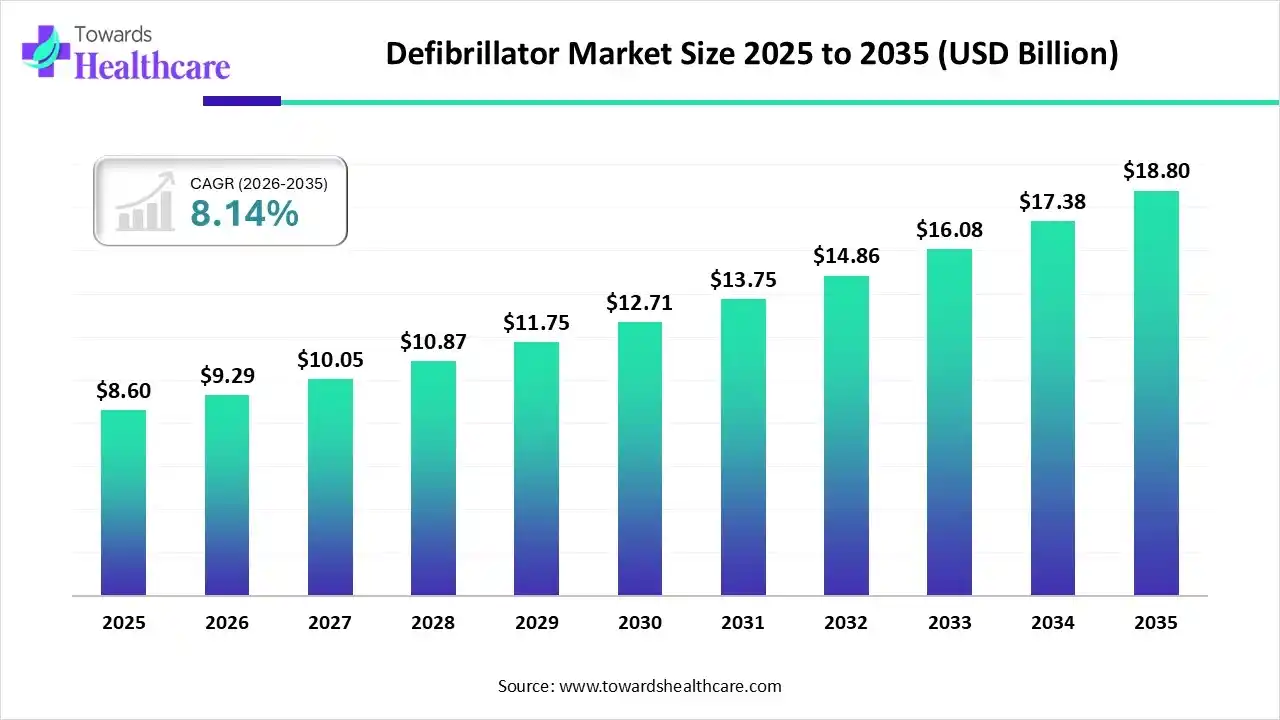
The global defibrillator market size was estimated at USD 8.6 billion in 2025 and is predicted to increase from USD 9.29 billion in 2026 to approximately USD 18.8 billion by 2035, expanding at a CAGR of 8.14% from 2026 to 2035.
Recent Developments in the Defibrillator Market
- In January 2026, Medtronic commercially launched the OmniaSecure defibrillation lead in the USA, with the first cases being performed at hospitals across the country.
- In December 2025, CHELMSFORD, MASS. ZOLL, an Asahi Kasei company that manufactures medical devices and related software solutions, announced the U.S. launch of the next-generation LifeVest wearable cardioverter defibrillator (WCD).
- In September 2025, Safe Life, a leading provider of life-saving solutions with operations across North America, Europe, and Asia, announced the acquisition of AED Brands, a Georgia-based company specializing in sales of automated external defibrillators (AEDs), consumables for AEDs, and services related to life-saving readiness.
Become a valued research partner with us, please feel free to contact us at sales@towardshealthcare.com
Keypoints
- Company Overview
- Locations Subsidiaries/Geographic reach
- Key Executives
- Company Financials
- Patents registered
- SWOT Analysis
- Applications Catered
- Strategic collaborations
- Recent Developments
- Competitive Benchmarking
