Explore Top Companies in the U.S. Antibody Specificity Testing Market 2026

Latest Updates of Key Players:
Danaher
- Headquarters: United States
- Latest Update: In May 2024, Danaher Corporation partnered with AstraZeneca to develop and commercialize innovative diagnostic tools and tests. These solutions are designed to help clinicians more accurately identify patients who would benefit most from precision medicine treatments.
Thermo Fisher Scientific Inc.
- Headquarters: United States
- Latest Update: In November 2025, Thermo Fisher Scientific Inc., a global leader in serving science, announced 510(k) clearance for the EXENT Analyser and Immunoglobulin Isotypes (GAM) Assay. This automated platform combines enhanced sensitivity and automation to deliver precise results, enabling faster diagnosis for multiple myeloma and related disorders.
Bio-Rad Laboratories, Inc.
- Headquarters: United States
- Latest Update: Bio-Rad expanded its portfolio with recombinant monoclonal anti-idiotypic antibodies and SpyCatcher reagents, strengthening support for bioanalytical assay development and antibody drug research.
Merck KGaA
- Headquarters: Germany
- Latest Update: In October 2025, Merck KGaA partnered with Promega Corporation, a US-based global life sciences solutions provider, to co-develop innovative technologies that advance drug screening and discovery.
Cell Signaling Technology, Inc. (CST)
- Headquarters: United States
- Latest Update: In 2025, CST continued to focus on rigorous antibody validation and ensuring lot-to-lot consistency, reinforcing its commitment to high-quality research standards.
BD (Becton, Dickinson and Company)
- Headquarters: United States
- Latest Update: BD has significantly enhanced its capabilities in antibody specificity and validation through advanced spectral imaging technologies, strengthening its role in the life sciences market.
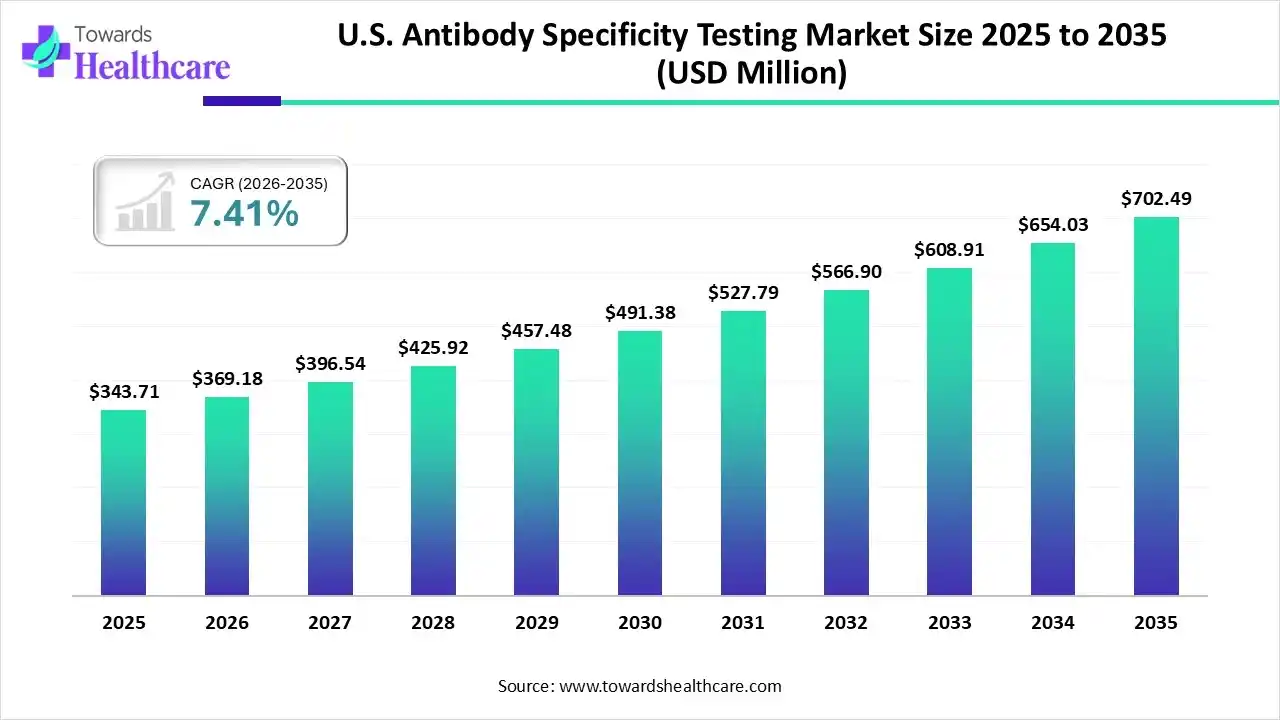
U.S. Antibody Specificity Testing Market Growth
The U.S. antibody specificity testing market size was estimated at USD 702.49 million in 2025 and is predicted to increase from USD 369.18 million in 2026 to approximately USD 702.49 million by 2035, expanding at a CAGR of 7.41% from 2026 to 2035.
Recent Developments in the U.S. Antibody Specificity Testing Market
- In October 2025, Harbour BioMed announced the launch of its first fully human Generative AI HCAb (Heavy Chain-Only Antibody) Model powered by its Hu-mAtrIx AI platform, built upon the Harbour Mice platform.
- In May 2025, CellFE, a leader in microfluidics-based cell engineering, and Made Scientific, a leading cell therapy contract development and manufacturing organization (CDMO), announced a strategic collaboration to generate pilot data on the CellFE High Volume Cyva System.
- In October 2025, Integral Molecular, a leader in antibody discovery and characterization, announced that its Membrane Proteome Array™ (MPA), used to assess antibody specificity, is entering its final stage of FDA review to become a Drug Development Tool (DDT). This follows Integral Molecular's submission of a Full Qualification Package (FQP) to the FDA.
Partner with our experts to explore the U.S. Antibody Specificity Testing Market at sales@towardshealthcare.com
Keypoints
- Company Overview
- Locations Subsidiaries/Geographic reach
- Key Executives
- Company Financials
- Patents registered
- SWOT Analysis
- Applications Catered
- Strategic collaborations
- Recent Developments
- Competitive Benchmarking
