February 2026

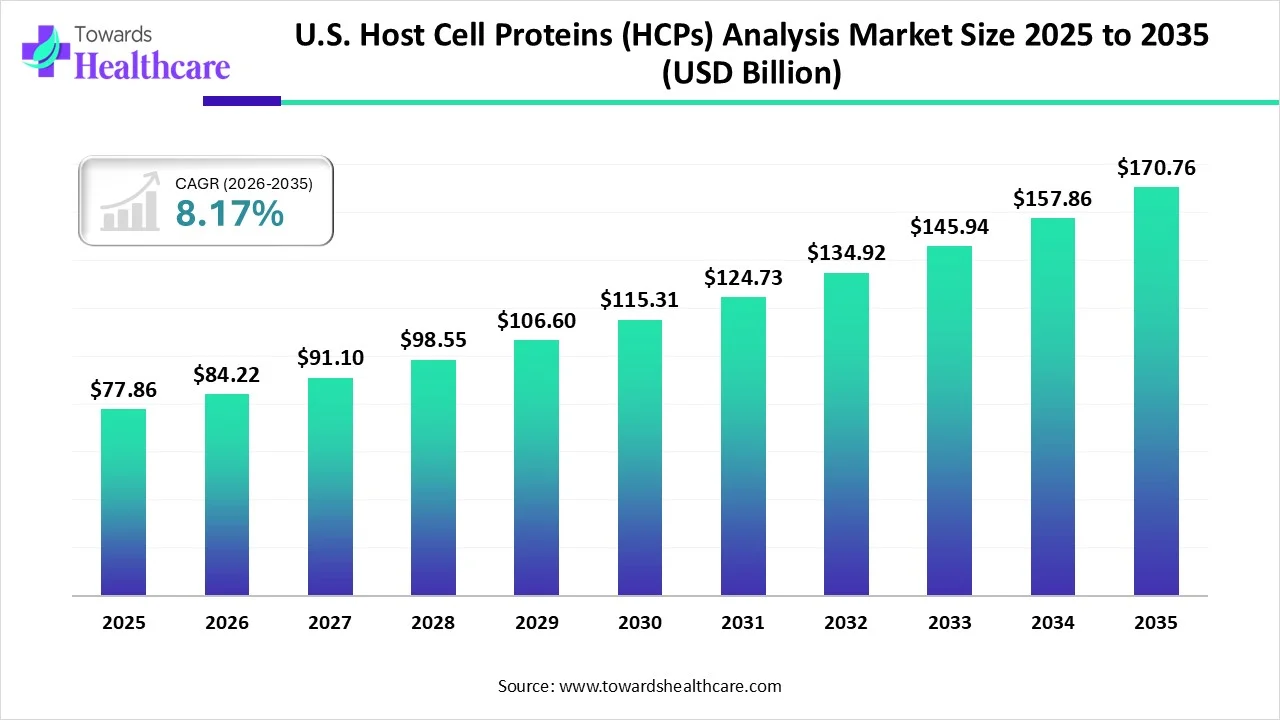
The U.S. host cell proteins (HCPs) analysis market size was estimated at USD 77.86 million in 2025 and is predicted to increase from USD 84.22 million in 2026 to approximately USD 170.76 million by 2035, expanding at a CAGR of 8.17% from 2026 to 2035.
The U.S. led the host cell proteins (HCPs) analysis market in 2205 by capturing the largest revenue share due to its strong concentration of biopharmaceutical companies, extensive biologics pipelines, and high manufacturing volumes. Stringent FDA regulatory requirements increased the need for rigorous HCP testing across development and commercial stages. Additionally, early adoption of advanced analytical technologies, strong CRO presence, and sustained R&D investments further reinforced the country’s market leadership.
Artificial intelligence can revolutionize the host cell proteins (HCPs) analysis market by enabling faster and more accurate data interpretation, predictive impurity profiling, and automated assay optimization. AI-driven analytics can enhance sensitivity in detecting low-level HCPs, reduce manual errors, and shorten development timelines. Additionally, machine learning models support real-time process monitoring, improving quality control efficiency and ensuring consistent regulatory compliance.
| Segmentations | Shares 2025 (%) |
| ELISA-based Assays | 38% |
| Mass Spectrometry (LC-MS/MS) | 30% |
| PCR-based Assays | 32% |
| Segmentations | Shares 2025 (%) |
| Biopharmaceutical Manufacturing | 25% |
| Quality Control (QC) | 35% |
| Cell & Gene Therapy | 40% |
| Segmentations | Shares 2025 (%) |
| Biopharmaceutical Companies | 60% |
| Contract Research Organizations (CROs) | 40% |
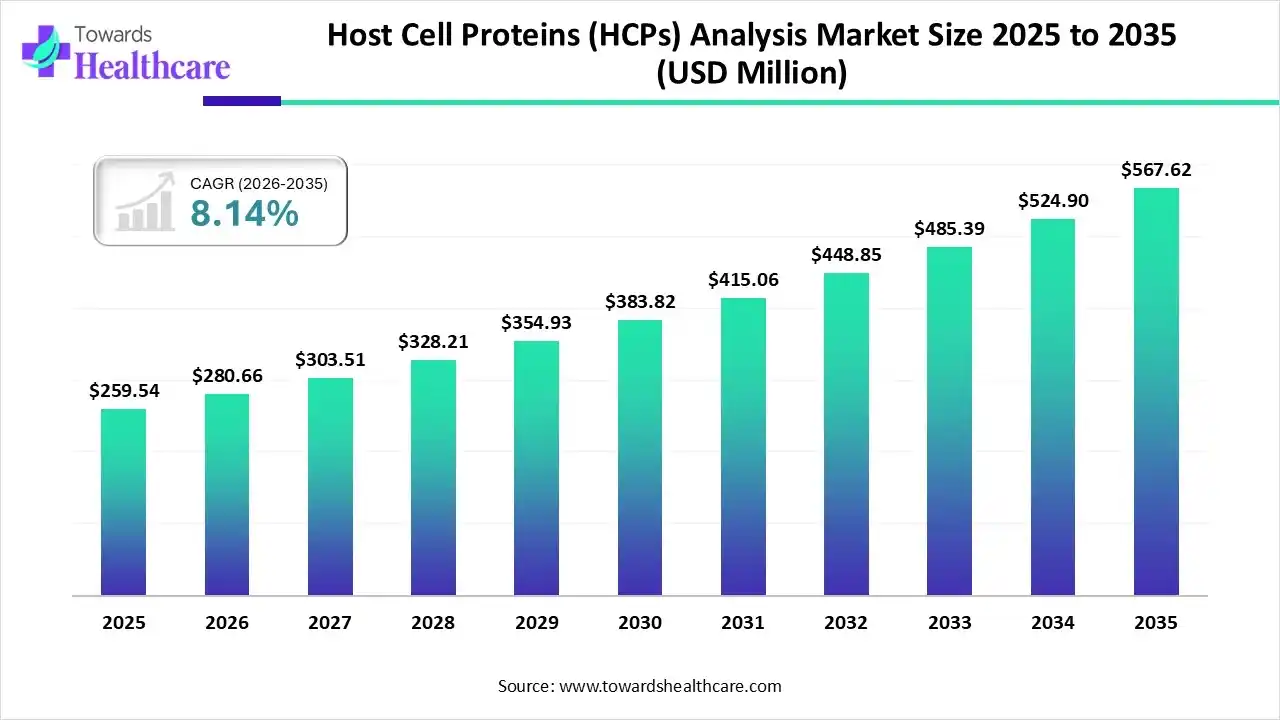
The global host cell proteins (HCPs) analysis market size was estimated at USD 259.54 million in 2025 and is predicted to increase from USD 280.66 million in 2026 to approximately USD 567.62 million by 2035, expanding at a CAGR of 8.14% from 2026 to 2035.
| Companies | Headquarters | Offering |
| Cygnus Technologies (Maravai LifeSciences) | California, US | Platform-specific and custom HCP ELISA kits, orthogonal impurity testing. |
| Thermo Fisher Scientific Inc. | Massachusetts, US | HCP ELISA kits, LC-MS/MS instruments, and integrated HCP analysis workflows. |
| Bio-Rad Laboratories, Inc. | California, US | HCP ELISA kits, immunoassay reagents, and QC testing solutions. |
| Charles River Laboratories | Massachusetts, US | HCP testing services, custom ELISA development, and MS-based HCP profiling. |
By Technology
By Application
By End-User
February 2026
February 2026
February 2026
January 2026