February 2026

The global iPSC cell therapies market size was estimated at USD 10.44 billion in 2025 and is predicted to increase from USD 11.64 billion in 2026 to approximately USD 30.87 billion by 2035, expanding at a CAGR of 11.45% from 2026 to 2035.
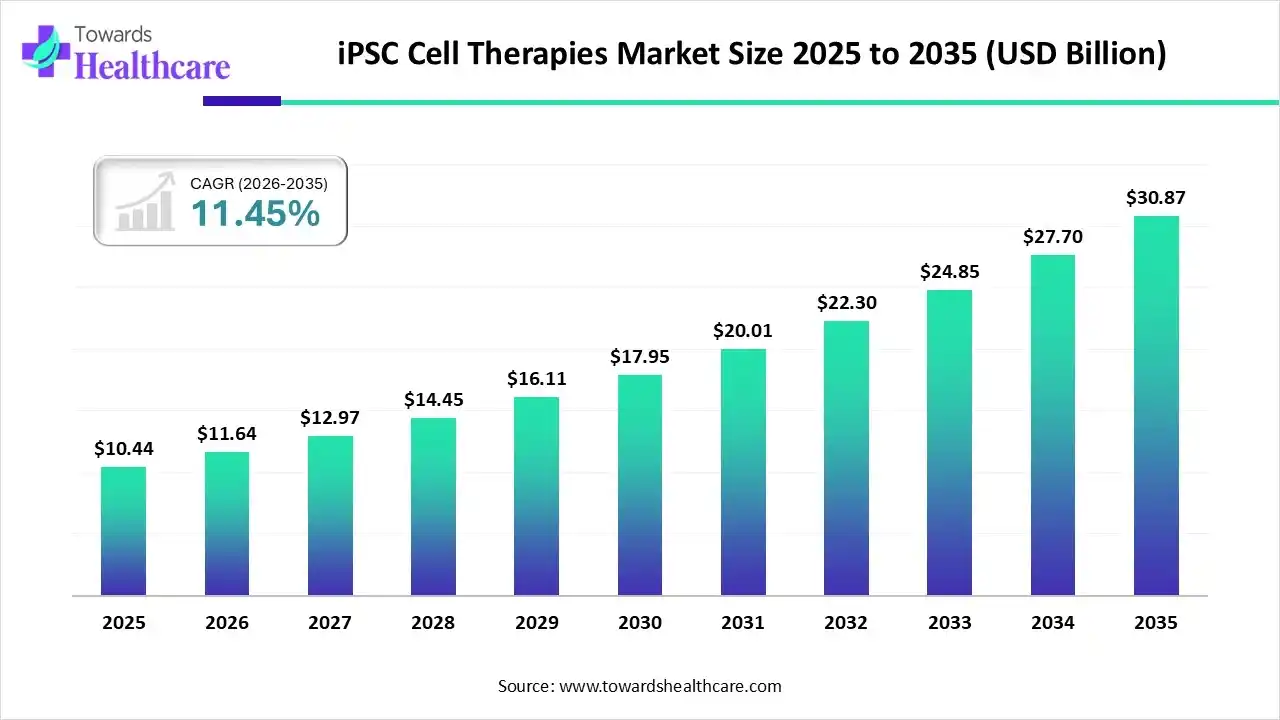
The industry has transitioned from a purely research-driven field into a clinical reality, with the world’s first regulatory submissions for iPSC-based therapies now occurring in Japan and the U.S. Additionally, growing disease burden, AI integration, and new product launches are also promoting the market growth.
The iPSC cell therapies market is driven by growing chronic diseases, R&D funding, and technological advancements. The iPSC cell therapies refer to the therapies utilizing induced pluripotent stem cells (iPSCs) for the treatment or repair of damaged tissue or organs. They are used in gene therapy, tissue regeneration, disease modelling, personalized treatments, and drug testing.
AI provides a wide range of applications in the market, such as analysis of large datasets, enhancing cell quality, and reducing errors. It also detects the cell growth, its health, abnormalities, and differentiates them, with the use of automated imaging and monitoring. It also detects drug response, toxicity, optimizes various manufacturing parameters, and helps in the development of personalized therapies, using patient health data.
The growing advancements in iPSC cell therapies are driving the development of new treatment options for various metabolic, retinal, rare genetic disorders, and autoimmune disorders.
The growing health awareness and focus on the development of patient-specific treatment are increasing the demand and development of various personalized autologous iPSC cell therapies with low rejection risks.
The companies are integrating advanced technologies such as CRISPR and other gene editing tools to develop novel curative therapies that can offer correction of genetic defects before transplantation.
| Key Elements | Scope |
| Market Size in 2026 | USD 11.64 Billion |
| Projected Market Size in 2035 | USD 30.87 Billion |
| CAGR (2026 - 2035) | 11.45% |
| Leading Region | North America |
| Market Segmentation | By Therapy Type, By Derived Cell Type, By Application, By End-User, By Region |
| Top Key Players | FUJIFILM Cellular Dynamics (FCDI), BlueRock Therapeutics (Bayer), Fate Therapeutics, Cynata Therapeutics, Astellas Pharma (Ocata), Evotec SE, Lonza Group AG, REPROCELL Inc., Aspen Neuroscience |
Why Did the Allogeneic iPSC Therapies Segment Dominate in the iPSC Cell Therapies Market in 2025?
The allogeneic iPSC therapies segment held the largest share of approximate 65% in the market in 2025, as they are favored for scalability and lower "off-the-shelf" costs. It also provided enhanced compliance with regulatory standards and improved batch consistency, which increased the quality of the products, which contributed to their increased use.
Autologous iPSC Therapies
The autologous iPSC therapies segment is expected to show the highest growth during the predicted time, as they are used for personalized neurology (e.g., Parkinson's) to avoid immune rejection. Moreover, the target-specific action, with low side effects and expanding applications are also increasing the adoption rates.
How Cardiomyocytes Segment Dominated the iPSC Cell Therapies Market in 2025?
The cardiomyocytes segment led the market with approximate 29% share in 2025, due to the growth in cardiovascular disease. This increased the demand for regenerative treatment options, which increased their use. Moreover, their clear functional endpoints also increased their demand and adoption rates.
Neurons/Neural Progenitors
The neurons/neural progenitors segment is expected to show the fastest growth rate during the predicted time, due to their growing use in Parkinson’s and spinal cord injury trials. At the same time, growing neurodegenerative diseases and the low self-repair capacity of the neurons are also increasing their use in the development of new treatment options.
Which Application Type Segment Held the Dominating Share of the iPSC Cell Therapies Market in 2025?
The drug discovery & toxicology segment held the dominating share of approximately 39% in the market in 2025, as the iPSC-derived cells were used to replace animal testing. They accelerated the R&D study and promoted their faster adoption. Furthermore, their affordability and scalability also encouraged their use.
Regenerative Medicine
The regenerative medicine segment is expected to show the highest growth during the upcoming years, as the iPSC cell therapies provide direct tissue replacement for AMD and heart failure. Moreover, the growing chronic diseases and expanding pipeline are also increasing their demand as a curative treatment option.
What Made Pharmaceutical & Biotech Companies the Dominant Segment in the iPSC Cell Therapies Market in 2025?
The pharmaceutical & biotech companies segment led the market with approximate 48% share in 2025, due to the growth in the R&D activities. Moreover, the growth in R&D investments and the expansion of the manufacturing infrastructure also increased their use in the development of various drug development processes.
CROs & CDMOs
The CROs & CDMOs segment is expected to show the fastest growth rate during the upcoming years, due to a surge in outsourcing of GMP-grade iPSC production. They also offer high technical expertise and affordable services, which lead to new collaborations, enhancing the development of iPSC cell therapies.
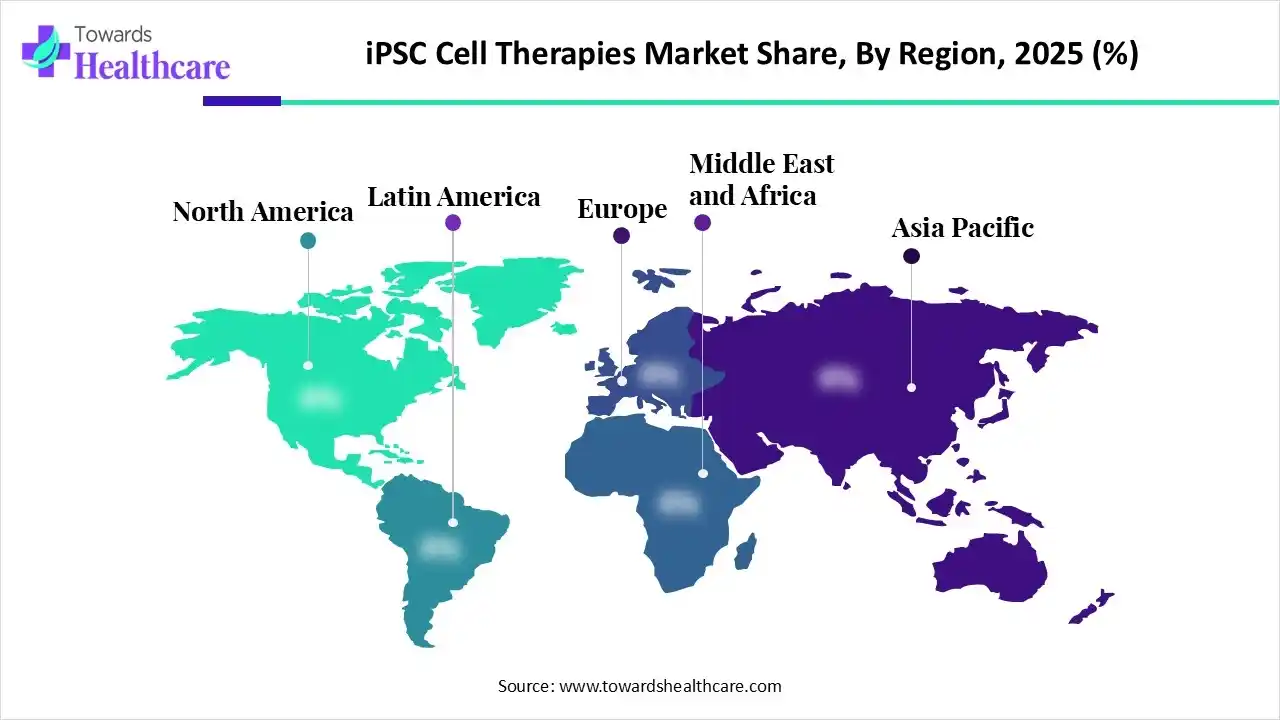
North America dominated the iPSC cell therapies market in 2025, due to the presence of robust R&D ecosystems. The growth in healthcare investments and funding also increased the development of iPSC cell therapies. Additionally, early adoption of advanced technologies, stringent regulations, and growth in clinical trials also contributed to the market growth.
U.S. Market Trends
The growth in healthcare investments and the presence of robust research institutes and universities in the U.S. are increasing the advancements in iPSC cell therapies. The growing regulatory approvals are also increasing their adoption, where the growing technological advancements are also accelerating their development and innovations.
Asia Pacific is expected to host the fastest-growing iPSC cell therapies market during the forecast period, due to expanding biotech hubs. The growing government support and chronic diseases are also increasing the demand for iPSC cell therapies. Moreover, growing R&D activities, collaborations, and expanding healthcare are also enhancing the market growth.
China Market Trends
China is experiencing a rapid growth in biotech startups and manufacturing capabilities, which are actively contributing to the development of new iPSC cell therapies. The growing government initiatives and increasing patient population are also increasing their innovation and adoption rates, respectively.
Europe is expected to grow significantly in the iPSC cell therapies market during the forecast period, due to growing clinical trials. The presence of advanced research infrastructures and regulatory bodies are also encouraging their use and driving their advancements. Furthermore, growing collaborations, R&D activities, and government grants are also promoting the market growth.
UK Market Trends
The presence of robust research infrastructure in the UK is increasing the iPSC-based R&D activities, driving the development of new iPSC cell therapies. The increasing approval and government funding are also increasing their advancements, where the growing partnerships are also increasing their use in the development of personalized solutions.

| Companies | Headquarters | iPSC Cell Therapies |
| FUJIFILM Cellular Dynamics (FCDI) | Madison, U.S. | Cardiology and neurology therapies |
| BlueRock Therapeutics (Bayer) | Boston, U.S. | Therapies for Parkinson’s disease, ophthalmology, and heart failure |
| Fate Therapeutics | San Diego, U.S. | Allogeneic NK CAR-T cell therapies |
| Cynata Therapeutics | Melbourne, Australia | Provides the Cymerus platform |
| Astellas Pharma (Ocata) | Tokyo, Japan | Regenerative ophthalmology via AIRM |
| Evotec SE | Hamburg, Germany | Provides an end-to-end iPSC platform |
| Lonza Group AG | Basel, Switzerland | Supports large-scale expansion and differentiation of iPSCs |
| REPROCELL Inc. | Yokohama, Japan | Provides StemRNA technology |
| Century Therapeutics | Philadelphia, U.S. | NK and T-cell therapies |
| Aspen Neuroscience | San Diego, U.S. | Personalized autologous iPSC-derived neuron replacement for Parkinson's disease |
By Therapy Type
By Derived Cell Type
By Application
By End-User
By Region
February 2026
January 2026
January 2026
January 2026