January 2026

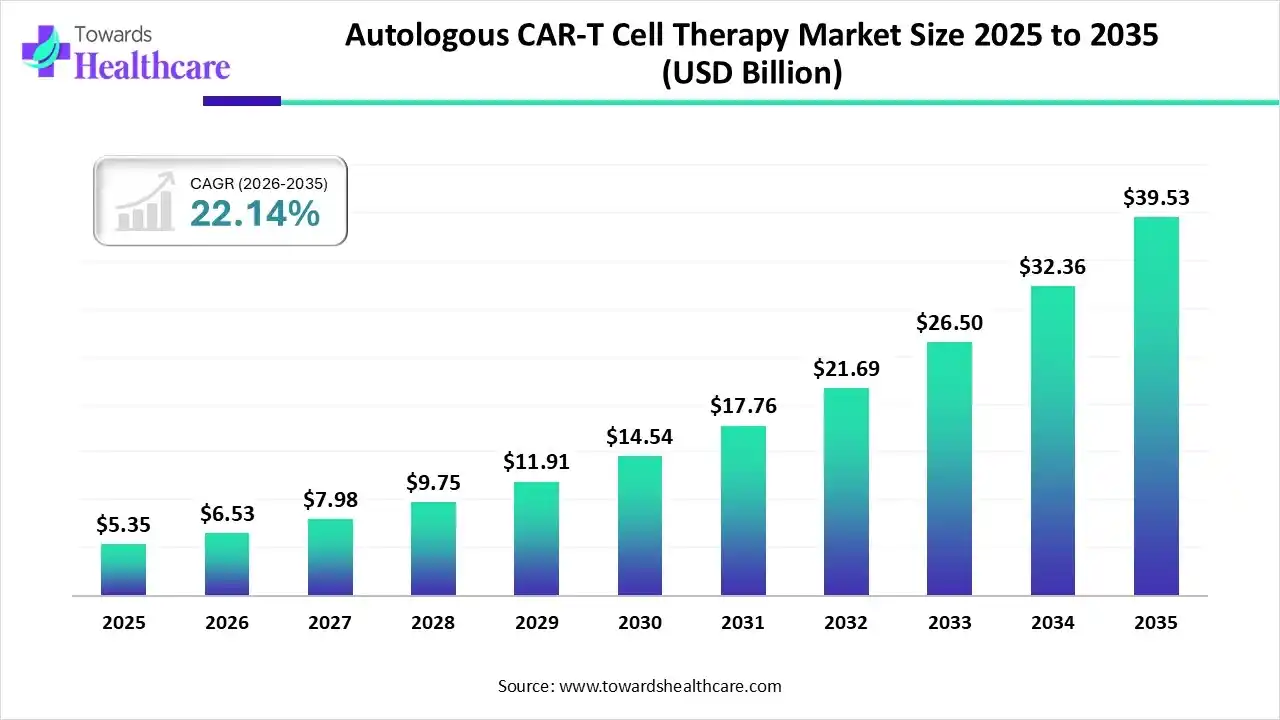
The global autologous CAR-T cell therapy market size was estimated at USD 5.35 billion in 2025 and is predicted to increase from USD 6.53 billion in 2026 to approximately USD 39.53 billion by 2035, expanding at a CAGR of 22.14% from 2026 to 2035.
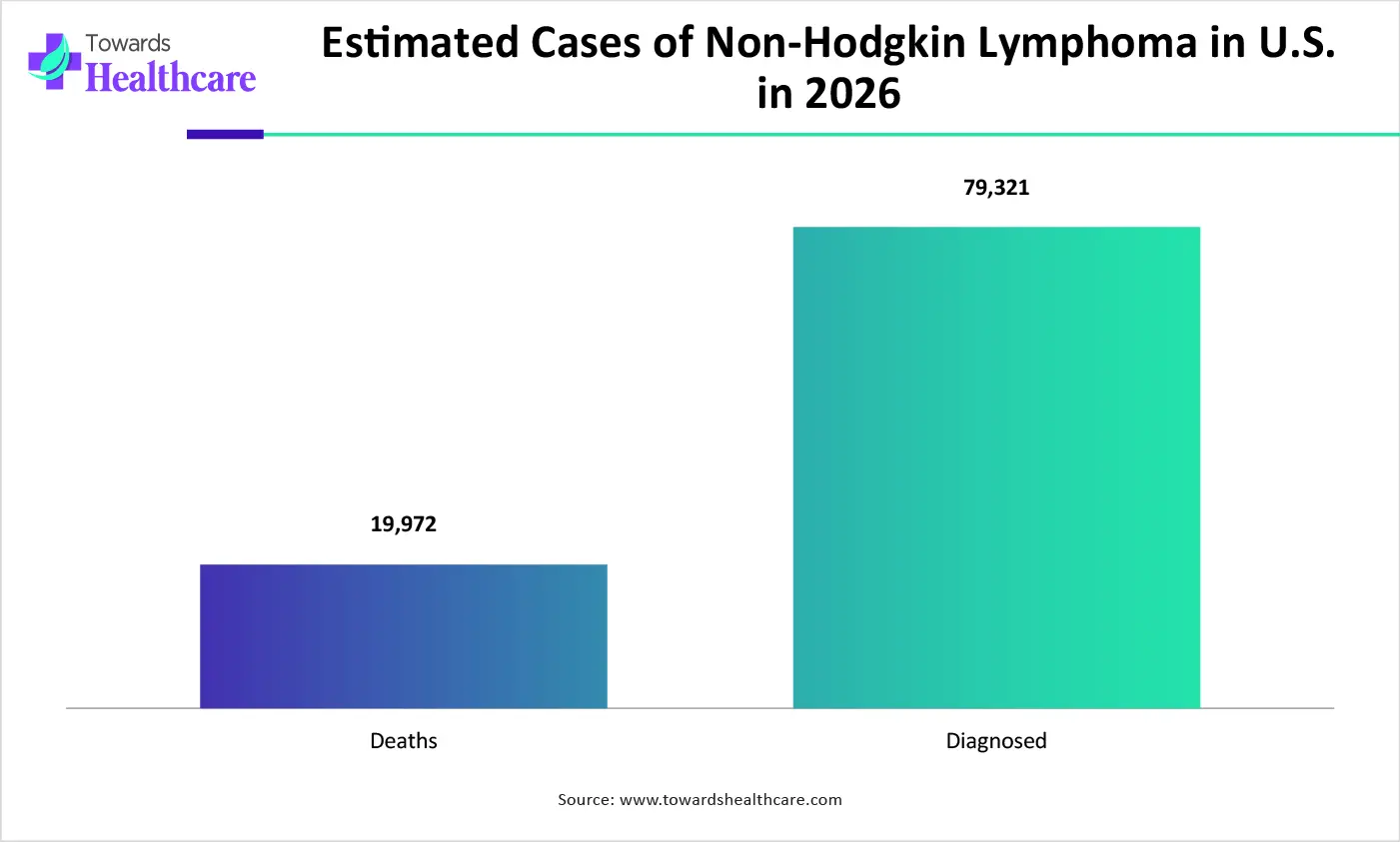
The growing incidence of cancer globally is increasing the demand for autologous CAR-T cell therapies. This is driving their innovations, development, AI integration, and launches, promoting market growth.
The autologous CAR-T cell therapy market is driven by increasing hematologic malignancies and expanding regulatory approvals. The autologous CAR-T cell therapy refers to the cancer immunotherapy, developed by genetically modifying the patient's T-cells to identify and target the cancer cells in the body. These therapies are being used in the treatment of different types of hematologic cancers.
There is a rise in the use of AI in the autologous CAR-T cell therapy market for the identification of the target, which helps in the development of these therapies with target-specific action. They also help in the optimization, manufacturing, and designing of next-generation therapies. It is also being used for the management of their toxicity and dosage, to enhance their safety and efficacy.
Due to the proven efficacy of the autologous CAR-T cell therapies in the treatment of hematologic cancer, their application in the treatment of other cancer types is increasing, promoting their R&D activities.
Different types of automated and closed system bioreactors are being developed, which are enhancing the manufacturing process of the autologous CAR-T cell therapies, enhancing their production rates.
The companies are focusing on the development of autologous CAR-T cell therapies with enhanced safety and efficacy, as well as developing novel drug delivery technologies, to enhance their target-specific action.
| Key Elements | Scope |
| Market Size in 2026 | USD 6.53 Billion |
| Projected Market Size in 2035 | USD 39.53 Billion |
| CAGR (2026 - 2035) | 22.14% |
| Leading Region | North America |
| Market Segmentation | By Product Type, By Application, By End-Use, By Therapy Development Phase, By Region |
| Top Key Players | Novartis, Gilead Sciences, Bristol Myers Squibb, Janssen, Autolus Therapeutics, ImmunoACT, Legend Biotech, CARsgen Therapeutics, JW Therapeutics, bluebird bio |
Why Did the CD19-Directed CAR-T Cell Therapy Segment Dominate in the Market in 2025?
The CD19-directed CAR-T cell therapy segment led the autologous CAR-T cell therapy market in 2025, due to its targeting specific action on B-cell leukemias. Their enhanced success rates also increase their use, where the growth in the patient volume also increases their demand and adoption rates.
BCMA-Directed CAR-T Cell Therapy
The BCMA-directed CAR-T cell therapy segment is expected to show the highest growth during the upcoming years, due to its growing success rates. Additionally, increasing interest in multiple myeloma treatment is also increasing their use and innovations, where their growing pipeline is also driving their demand.
How Acute Lymphoblastic Leukemia Segment Dominated the Market in 2025?
The acute lymphoblastic leukemia segment held the dominating share in the autologous CAR-T cell therapy market in 2025, driven by the proven success rates of the autologous CAR-T cell therapies. Moreover, their target-specific action on CD19 expression and increased approvals also enhanced their use.
Chronic Lymphocytic Leukemia
The chronic lymphocytic leukemia segment is expected to show rapid growth during the upcoming years, due to growing incidence rates. This is increasing the use of autologous CAR-T cell therapies, where their expanding pipeline and the development of combination therapies are also increasing their acceptance rates.
What Made Hospitals the Dominant Segment in the Market in 2025?
The hospitals segment led the autologous CAR-T cell therapy market in 2025, due to growth in patient volumes. The presence of well-developed infrastructure and reimbursement policies also attracted the patients. Moreover, they also offered continuous monitoring and compliance with regulatory standards, which increased the patient outcomes.
Specialty Clinics
The specialty clinics segment is expected to show the highest growth during the predicted time, due to the growing number of outpatients. At the same time, they also offered affordable and advanced autologous CAR-T cell therapies, which attracted the patients. Additionally, flexible scheduling and skilled personnel are also increasing their preference.
Which Therapy Development Phase Type Segment Dominated the Market in 2025?
The preclinical segment held the dominating share in the autologous CAR-T cell therapy market in 2025, due to growth in the autologous CAR-T cell candidates. The growth in the R&D activities in institutes and industries also increased their participation in this phase to evaluate the product's safety, applications, and efficacy.
Phase II
The phase II segment is expected to show rapid growth during the predicted time, due to the rapid expansion of the autologous CAR-T cell therapy pipeline. The companies are actively participating in this phase to enhance and accelerate the launch of their products with different types of applications.
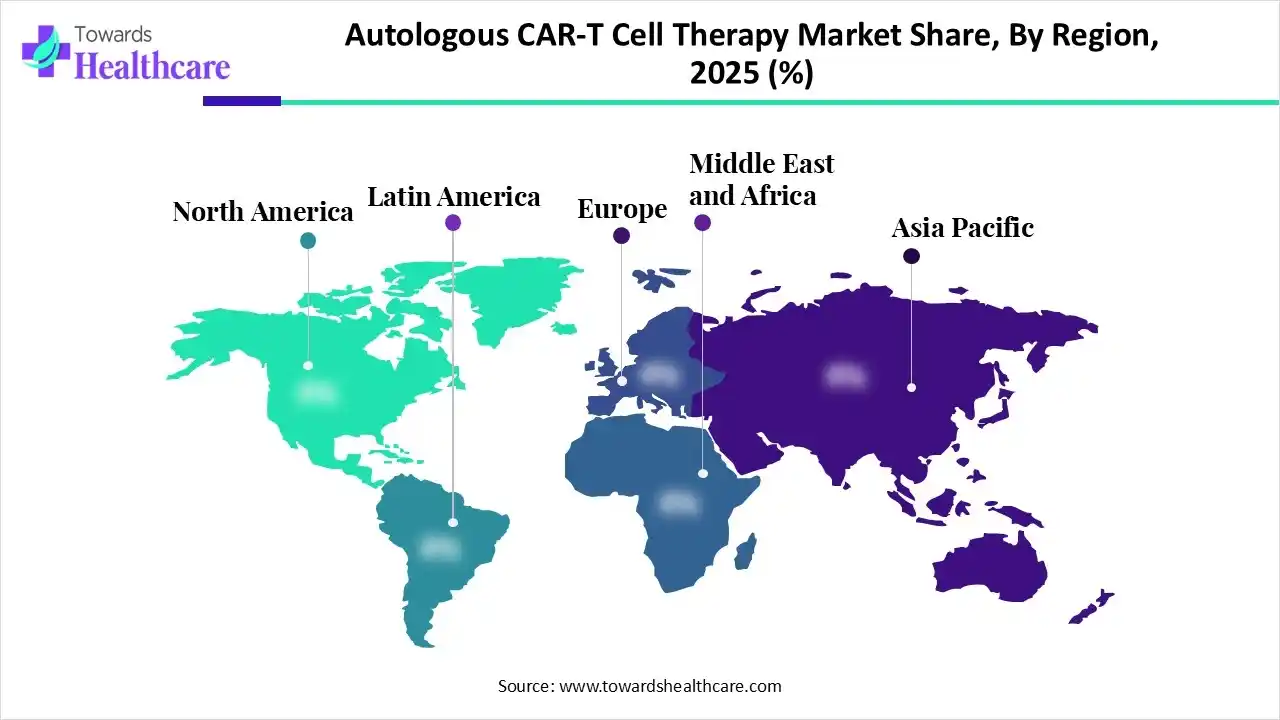
North America dominated the autologous CAR-T cell therapy market in 2025, due to the presence of a well-developed R&D infrastructure. This increased the development and innovations of the autologous CAR-T cell therapies, along with their early adoption. Moreover, the presence of advanced healthcare systems also increased their adoption and approval rates, which contributed to the market growth.
U.S. Market Trends
The presence of advanced industries and healthcare in the U.S. is increasing the development of autologous CAR-T cell therapies. The growing healthcare investments and expanding applications are also increasing their innovations, where the faster approval rates are also encouraging their advancements.
Asia Pacific is expected to host the fastest-growing autologous CAR-T cell therapy market during the forecast period, due to the growth in cancer cases. The expanding healthcare sector is also increasing the adoption of advanced treatment options, like autologous CAR-T cell therapies. Furthermore, growing government support and increasing R&D activities are also enhancing their innovations, promoting market growth.
China Market Trends
Due to the presence of a large population, there is an increase in the number of patients with lymphoma, leukaemia, and myeloma in China, which is increasing the use of autologous CAR-T cell therapies. Moreover, the expanding biotechnology ecosystem and its application are also increasing their development, which is backed by new investments.
Europe is expected to grow significantly in the autologous CAR-T cell therapy market during the forecast period, due to the presence of advanced industries and the healthcare sector. This is increasing their production, innovations, and availability, enhancing their adoption rates. Additionally, growing health awareness is also increasing their demand, enhancing the market growth.
UK Market Trends
The UK consists of well-developed healthcare systems, which increases the use of autologous CAR-T cell therapies for the treatment of growing cases of lymphoma. The presence of reimbursement policies is also increasing their use and accessibility, which is increasing R&D activities, driving their innovations.

| Companies | Headquarters | Autologous CAR-T Cell Therapies |
| Novartis | Basel, Switzerland | Kymriah |
| Gilead Sciences | California, U.S. | Tecartus and Yescarta |
| Bristol Myers Squibb | New Jersey, U.S. | Abecma and Breyanzi |
| Janssen | New Jersey, U.S. | Carvykti |
| Autolus Therapeutics | London, UK | Aucatzyl |
| ImmunoACT | Navi Mumbai, India | NexCAR19 |
| Legend Biotech | New Jersey, U.S. | Carvykti |
| CARsgen Therapeutics | Shanghai, China | Zenvorcabtagene autoleucel |
| JW Therapeutics | Shanghai, China | Relmacabtagene autoleucel |
| bluebird bio | Massachusetts, U.S. | Abecma |
By Product Type
By Application
By End-Use
By Therapy Development Phase
By Region
January 2026
January 2026
January 2026
January 2026