

Siloam and AWS Cloud Entered a Partnership to Enhance Patient Care Announcement PT Siloam International Hospitals Tbk initially adopted cloud services involving initially from Amazon Web Services (AWS) to leverage AI-driven innovation and digital transformation in healthcare. With an almost n...
Medexus Wins this Quarter's Race with the Successful Debut of its New Drug Announcement Medexus Pharmaceuticals smartly achieved a modest revenue winner position in this quarter for its energized debut of its new drug GRAFAPEX. This new drug managed to recoup losses from generics eating into i...

Concentra, After a Steady 2024, is Back for Dealmaking with Many Biotech Firms Concentra Biosciences Comeback One of the busiest buyers of biotech has been suspicious. Concentra Biosciences, the popular biotech buyout operated by Tang Capital, has walked into a dealmaking after its slow and st...
Ocugen Closed the Registered Direct Offering at $20 Million Announcement Ocugen Inc., a leading biotechnology company in gene therapies for blindness diseases, closed its previously introduced registered direct offering regarding securities purchase agreement with Janus Henderson, a leading as...

Modular Medical’s Participation at the ADCES Announcement Modular Medical Inc., a leading insulin delivery technology company with its first-ever FDA-approved patch pump introduced mainly to target all adult ‘almost-pumpers’ with its affordable and user-friendly aspect,...

Ocugen’s and Janus Securities' Purchase Agreement Announcement Ocugen Inc., a global biotechnology company in gene therapies for blindness diseases, signed a securities purchase agreement with Janus Henderson Investors, a leading asset management firm. Janus will purchase 20,000,000 sha...

Five Oral Obesity Candidates, a Competition to Lilly’s Orforglipron Lilly’s Orforglipron’ The Phase 3 data for Eli Lilly’s early oral GLP-1 therapy, orforglipron, were released last week. The addition of new advancements to the already advanced weight-loss space needs o...
Orchestra’s Rollout of the FDA-approved Protocol Update Announcement Orchestra Biomed Holdings, Inc., a leading biomedical company elevating excellent technologies to patients via risk-rewarded sharing partnerships, declared its official rollout of a US FDA protocol update. It has promin...
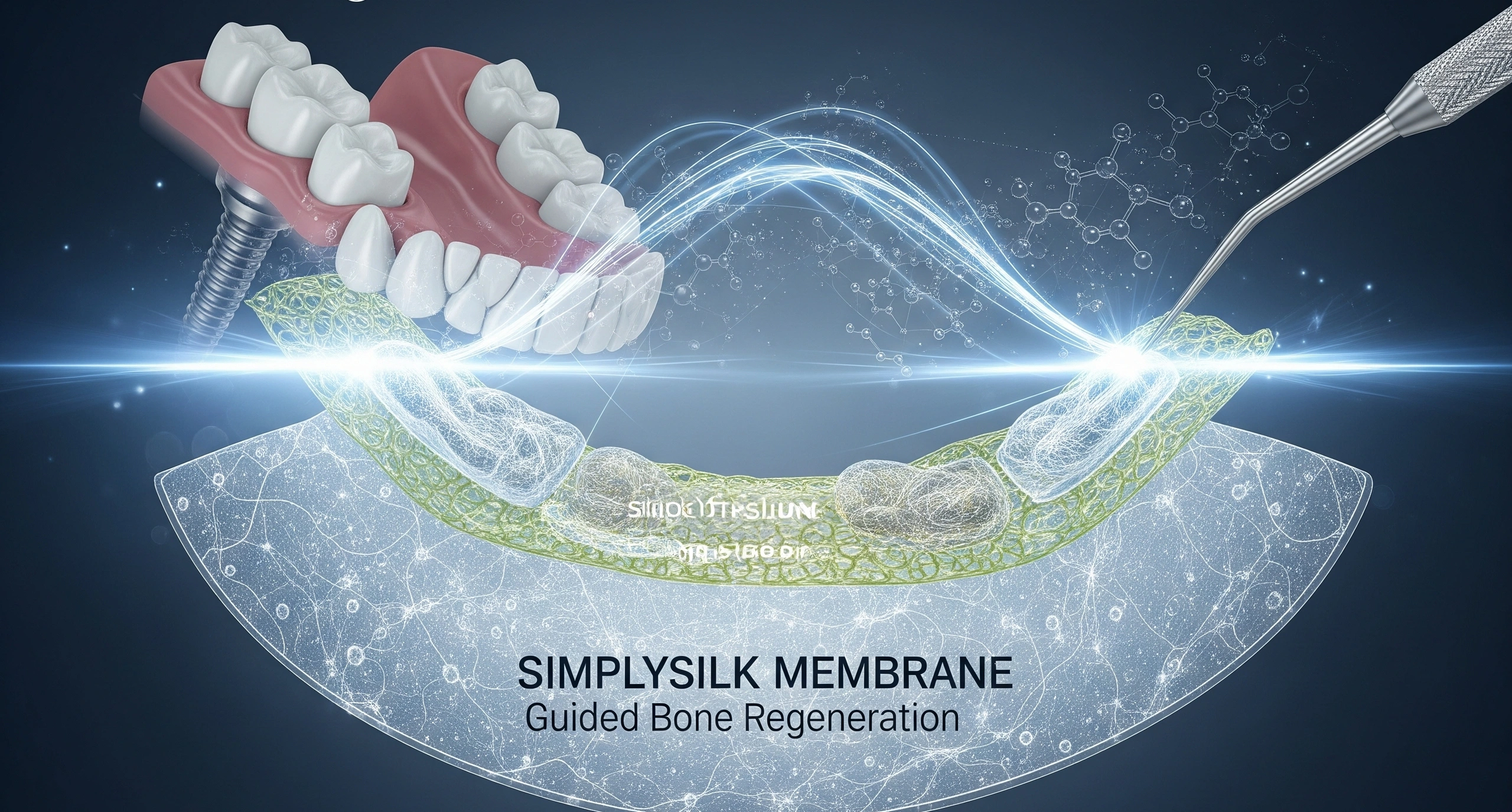
Fibrothelium and Vivolta Entered into a Manufacturing Contract Announcement Vivolta, a medical mechanism solution provider, entered a long-term contract with Fibrothelium, a pioneer in silk protein biomaterial. The partnership’s motive is to manufacture electro-driven components for use ...
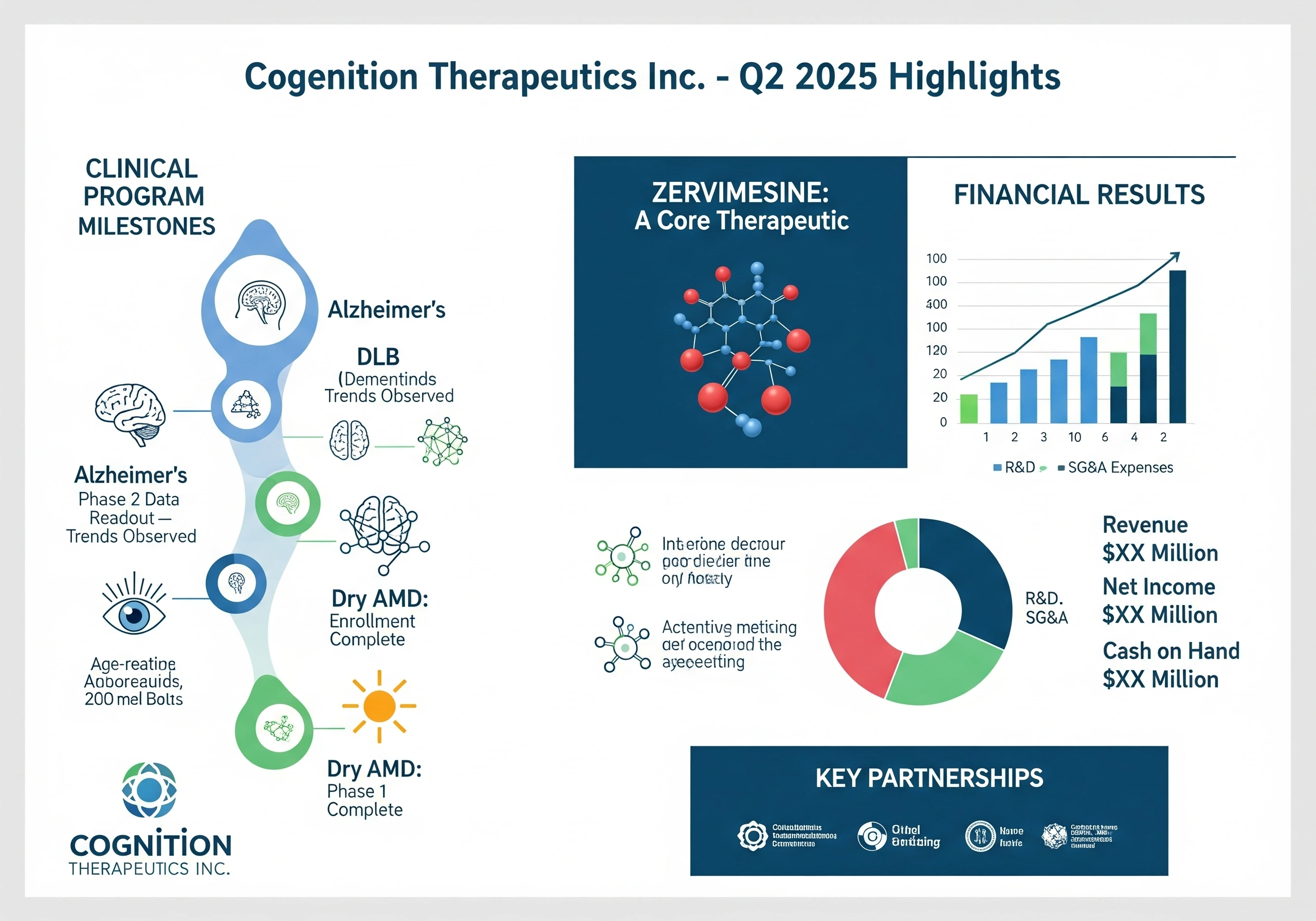
Cognition’s Progressive Clinical Programs and Financial Results for the Second Quarter of 2025 Announcement Cognition Therapeutics Inc., a leading clinical-stage company producing advanced drugs for the treatment of neurodegenerative disorders, declared its financial results for the seco...