January 2026

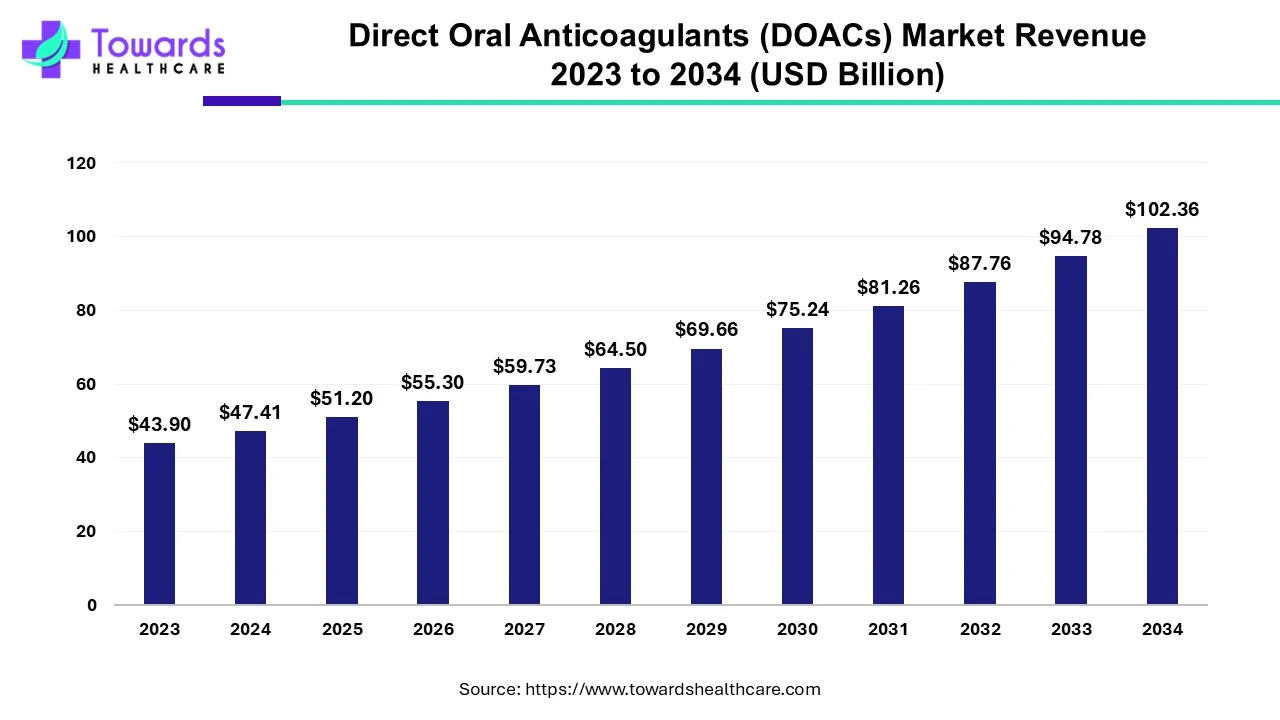
The global direct oral anticoagulants (DOACs) market was estimated at US$ 43.9 billion in 2023 and is projected to grow to US$ 102.36 billion by 2034, rising at a compound annual growth rate (CAGR) of 8% from 2024 to 2034. DOACs are in high demand due to their use as anticoagulation in various cardiovascular diseases.
Direct oral anticoagulants, or DOACs, have rapidly emerged as appealing substitutes for the established anticoagulation standard of therapy, which includes vitamin K antagonists. A number of cardiovascular disorders can be prevented and treated using DOACs. In thromboembolic situations, DOACs have become the most popular therapeutic substitutes, offering patients and physicians safer, more convenient, and more effective treatment options. Clinicians must make more difficult judgments about the right drugs, therapy length, and use in particular patients as a result of DOACs' growing involvement.
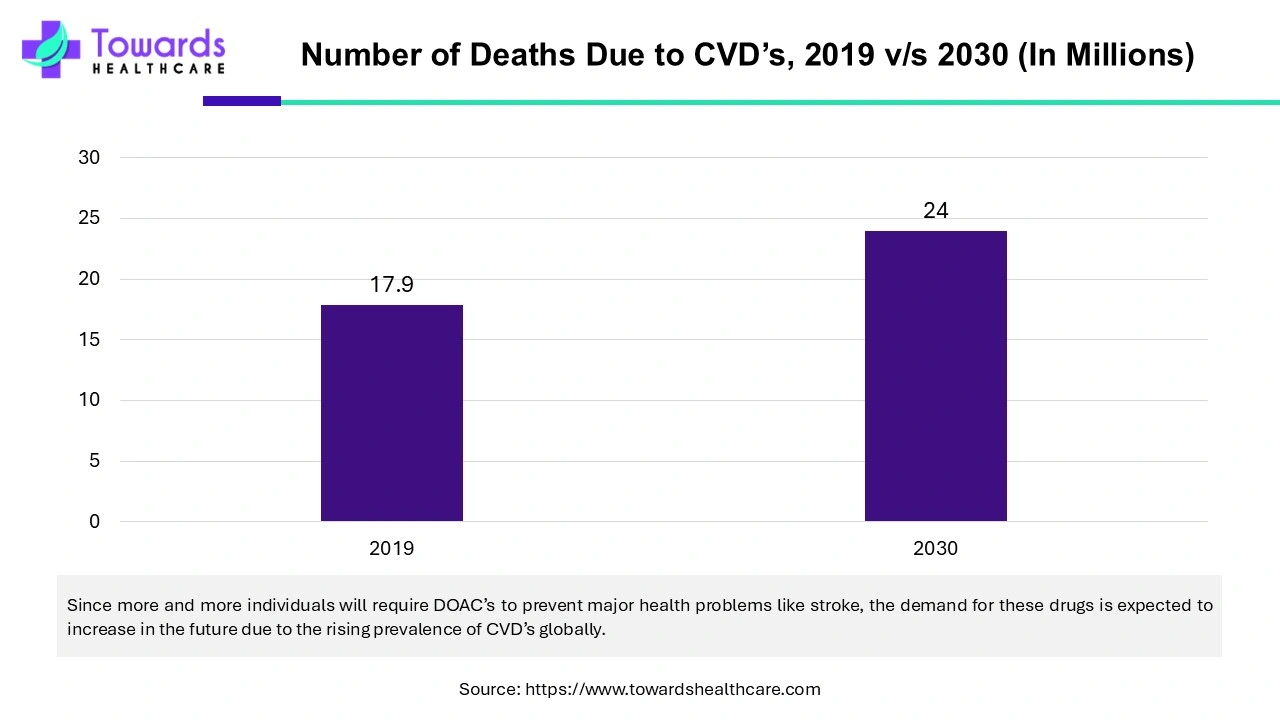
Despite improvements in disease prevention and treatment, cardiovascular diseases (CVDs) remain one of the top causes of death globally. The US economy is heavily impacted by NCDs, primarily CVDs, as the Centers for Disease Control and Prevention (CDC) noted in 2022. The healthcare system spends over $216 billion on NCDs, and the rising rate of workplace absenteeism and the resulting decreased productivity cost the US economy $147 billion. Healthcare expenses are severely impacted by thrombotic events in CVDs. The direct oral anticoagulants (DOACs) market is growing since anticoagulant usage is advised for the prevention of thrombotic events in a variety of cardiovascular conditions, such as stroke prevention in atrial fibrillation, therapy, and secondary prevention of acute coronary syndrome.
The direct oral anticoagulants (DOACs) market faces challenges due to the potential side effects of using DOACs. Like any drug, DOACs might have negative effects on certain persons. According to the patient information booklet that comes with your medication, various DOACs might have different adverse effects. Any anticoagulant may cause bleeding as a negative effect. Major bleeding is one of the more significant complications that can occur.
The major growth factor of the direct oral anticoagulants (DOACs) market is the growing research and development activities. This enables researchers to identify disease progression and develop novel molecules of the same or different classes. Researchers focus on developing novel drugs with enhanced efficacy and reduced side effects. The increasing use of artificial intelligence (AI) and machine learning (ML) aids in predicting the pharmacokinetic and pharmacodynamic properties of drug-like candidates, expediting the drug screening process. Key players are deriving generic alternatives to existing brand-name drugs. This increases the affordability and accessibility of DOACs among large population groups. They are also determining the extended applications of DOACs for diverse disorders.
The dominance of North America is due to various factors, including advanced healthcare infrastructure, government support, growing investments in pharmaceuticals, and innovation & investments by key market players. The two major countries that contribute to the market’s growth are the U.S. and Canada.
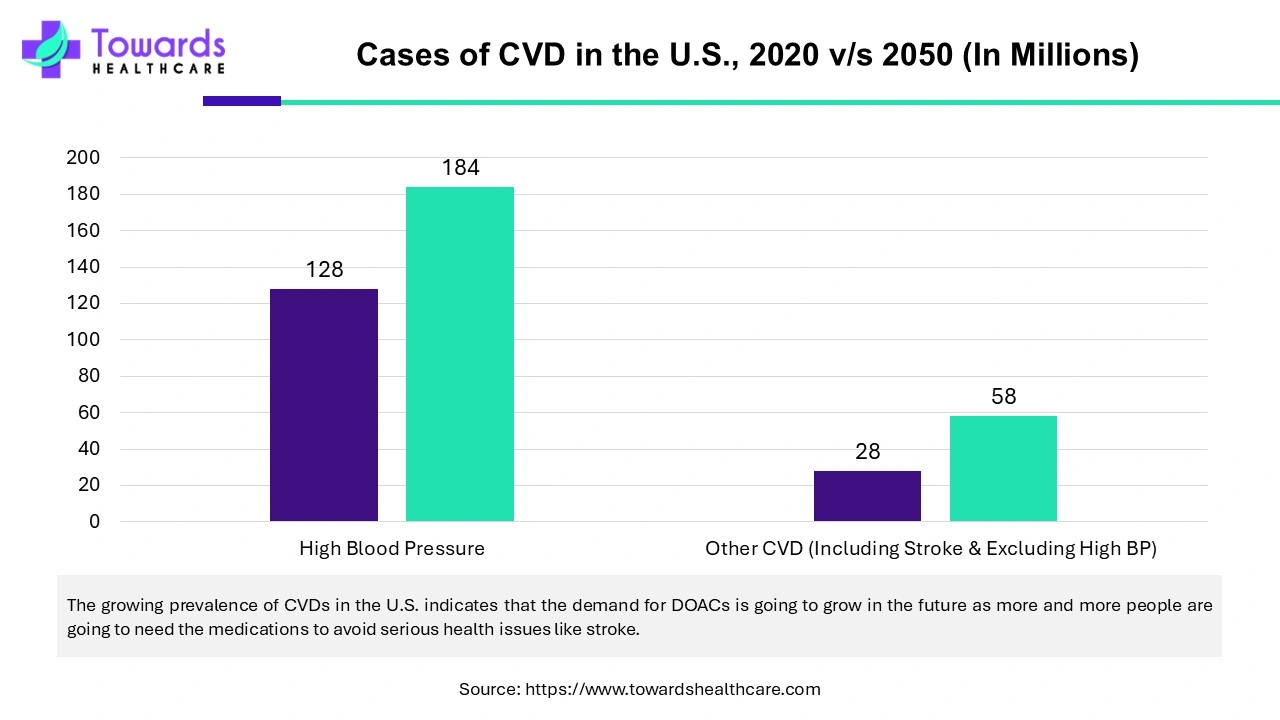
The U.S. dominated the direct oral anticoagulants (DOACs) market in North America. The rising incidence of cardiovascular disorders is the main cause of the increase. Governments and major industry participants are taking initiatives to address the prevalence of CVDs in the United States. It is estimated that the annual healthcare expenses of cardiovascular risk factors, adjusted for inflation in 2022 US dollars, will treble from $400 billion to $1344 billion between 2020 and 2050. It is anticipated that the yearly cost of healthcare for cardiovascular problems will nearly triple, from $393 billion to $1490 billion, and that productivity losses will rise by 54%, from $234 billion to $361 billion.
Asia Pacific is the largest region with a large population, which provides advantages by providing a large consumer base and a large manpower. The direct oral anticoagulants (DOACs) market is growing strongly in the region due to factors like innovation, investment in healthcare, and investments by international market players. Another major factor that contributes to the growth is the growing prevalence of CVDs in the region. Some of the countries that mainly contribute to the growth of the market include China and India. Japan, and South Korea.
There are an estimated 330 million CVD patients in China today, which includes 245 million cases of hypertension, 12.39 million cases of coronary heart disease, 8.9 million cases of heart failure, 5 million cases of pulmonary heart disease, 4.87 million cases of atrial fibrillation, 2.5 million cases of rheumatic heart disease, 2 million cases of congenital heart disease, 45.3 million cases of peripheral artery disease, and 13 million strokes.
The Government of India's Ministry of Health launched a public campaign on heart health in September 2023, today, World Heart Day. The campaign focuses on increasing awareness of the risk factors for hypertension, which can result in cardiovascular disease-related morbidity and early death. The government set the lofty goal of enrolling 75 million people with diabetes and hypertension in routine treatment by December 2025. It has already begun to show benefits and is billed as the largest primary healthcare initiative in the world. According to the National NCD Portal, 11 million people with diabetes and hypertension were receiving treatment as of August 2023.
By type, the oral factor Xa inhibitors segment dominated the market. This segment dominated because many nations have now approved new oral anticoagulants for certain clinical reasons that specifically block either thrombin (dabigatran etexilate) or factor Xa (rivaroxaban, apixaban). These medications, in contrast to warfarin, have a quick beginning of action and a broad therapeutic range. Therefore, coagulation monitoring is not necessary. In addition to being more cost-effective than current treatments, these agents have the potential to enhance clinical outcomes and are more convenient for both patients and healthcare professionals.
For instance,
By application, the atrial fibrillation segment held a significant share of the market. The most prevalent clinically relevant heart rhythm problem, atrial fibrillation (AF), is regarded as an epidemic of cardiovascular disease in the twenty-first century. According to projection studies, the number of people with AF is expected to increase to 15.9 million in America by 2050 and 17.9 million in Europe by 2060. Both North American and European recommendations advocate direct oral anticoagulants (DOACs) as the first-line treatment for atrial fibrillation patients in order to prevent ischemic stroke.
Dr. Hakim Yadi, CEO and Co-founder at Closed Loop Medicine, commented that establishing a strong and differentiated patent portfolio is central to the company’s leadership in AI-enabled personalized medicine. These patents reinforce the ability to deliver smarter, AI-enabled pharmacometric dosing solutions. (Source: https://www.news-medical.net/news/20250325/US-Patent-Office-grants-Closed-Loop-Medicine-key-patents-for-cardiometabolic-capabilities-in-GLP-1-and-Direct-Oral-Anti-Coagulants-(DOAC)-therapies.aspx)
By Type
By Application
By Region
January 2026
January 2026
November 2025
December 2025