February 2026

The global complement C4 antibody market was estimated at US$ 19.21 billion in 2023 and is projected to grow to US$ 68.48 billion by 2034, rising at a compound annual growth rate (CAGR) of 12.25% from 2024 to 2034.
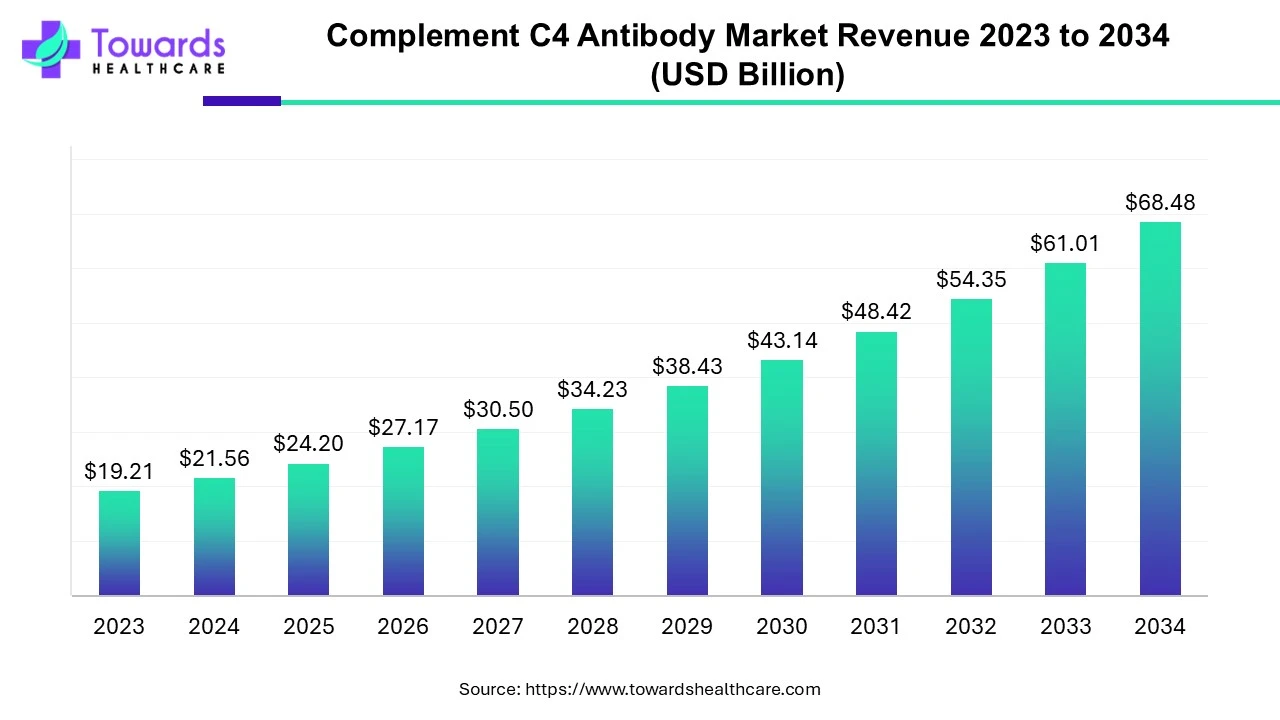
The complement C4 antibody market encompasses production, supply, research and development, and other aspects of complement C4 antibodies. These antibodies are developed for the complement C4, which is a component of the complement system. This system is an essential part of the immune response that kills pathogens, promotes inflammation, and enhances phagocytosis. The demand for antibodies is growing because researchers use antibodies to study the function of the complement system. These antibodies are also used for studying autoimmune diseases in which the complement system either regulates improperly or becomes overactive.
Autoimmune is a rising concern and needs attention from researchers and the healthcare system to provide better treatment options. The growing cases of autoimmune diseases create growth opportunities for the complement C4 antibody market because the market provides an opportunity for developing better treatment options.
Complementing C4 antibodies is essential in researching and diagnosing autoimmune conditions. The antibodies are used in understanding the role of the complement system in autoimmune diseases. The growing need for the complement C4 antibodies demands investments and innovation in the sector.
Production of complement C4 antibodies is a complex process that is expensive and time-consuming. During research, extensive lab work is done, which includes protein purification, culturing cells, and validation studies, which further increases the cost. A further cost is added due to the need for advanced technology, equipment, and skilled professionals. These costs can become a significant barrier for start-ups or smaller companies that are looking to enter the market.
North America Dominated the complement C4 antibody market in 2023. North America has a strong presence of key market players that collaborate with government organizations and other key players to conduct research and development. Apart from this complement, C4 antibodies are used by pharmaceutical and biotechnology companies for various purposes, and North America dominates in both sectors.
The U.S. held the largest share of the complement C4 antibody market in North America. The country strongly invests in research and development, developing new treatment options and other aspects of healthcare. Apart from this, there is a growing prevalence of autoimmune diseases in the U.S. According to the National Institute of Environmental Health Sciences, around 50 million people in the U.S. have an autoimmune disease. This makes autoimmune disease the third most prevalent disease category. The government and other organizations are making efforts in R&D and improvement of autoimmune disease treatment.
For instance,
Asia Pacific is expected to grow at the fastest rate during the forecast period. The complement C4 antibody market is growing in Asia Pacific because of growing government investments in research and development and the improvement of the healthcare system. China, India, Japan, and South Korea are the countries that majorly contribute to the market's growth. There is growing research in these countries related to autoimmune diseases.
For instance,
The government of India formed the National Consortium for Research and Development on Therapeutics for Rare Illnesses to encourage collaboration in the field of rare disease research and development for the purpose of diagnosing and treating rare illnesses in the country. A number of autoimmune disorders fall under the uncommon disease umbrella.
Europe is expected to grow significantly in the complement C4 antibody market during the forecast period. The industries in Europe are developing various new treatment options for different diseases using the complement C4 antibody. The rising occurrences of cancer, as well as other diseases, are increasing the use of complement C4 antibody treatment options. This further drives the production process. The innovation carried out using the complement C4 antibody for developing new treatment approaches in the industries of German industry contributes to the market growth.
By type, the monoclonal antibodies segment dominated the complement C4 antibody market and is expected to grow with the fastest CAGR during the forecast period. One of the most effective biologics for a range of uses is monoclonal antibodies. These compounds have great selectivity, specificity, and binding affinity, which are usually reasons to use them. Furthermore, the majority of monoclonal antibodies may be readily made in recombinant systems and have well-defined sequences, affinities, and other characteristics. Strong batch-to-batch consistency and excellent purity are ensured by producing these reagents in specified systems and media, which is crucial for many applications.
By application, the immunoassays segment held the largest complement C4 antibody market share in 2023 and is expected to grow rapidly during the forecast period. Applications for complement C4-detecting antibodies in science include immunohistochemistry, ELISA, and immunoprecipitation. Complement C4 is the target of these antibodies in samples from humans, mice, and rats. With the aid of complement C4 antibodies, immunoassays are utilized to discover health problems. A patient with symptoms suggestive of an accident, recent infection, or autoimmune illness may be advised to undergo the complement C4 test by their physician.
By end-user, the pharmaceutical & biotechnology companies segment showcased its dominance over the market in 2023. These companies use complement C4 antibodies for research and development, or developing diagnostic kits, and development of better treatment options. By end-user, the diagnostic laboratories are estimated to grow at the fastest CAGR in the complement C4 antibody market during the predicted timeframe. Complement C4 antibodies are used to conduct C4 complement tests. These tests are conducted in diagnostic laboratories for the detection of autoimmune disorders, injuries, or recent infections.
By Type
By Application
By End-User
By Region
February 2026
February 2026
January 2026
November 2025
