February 2026

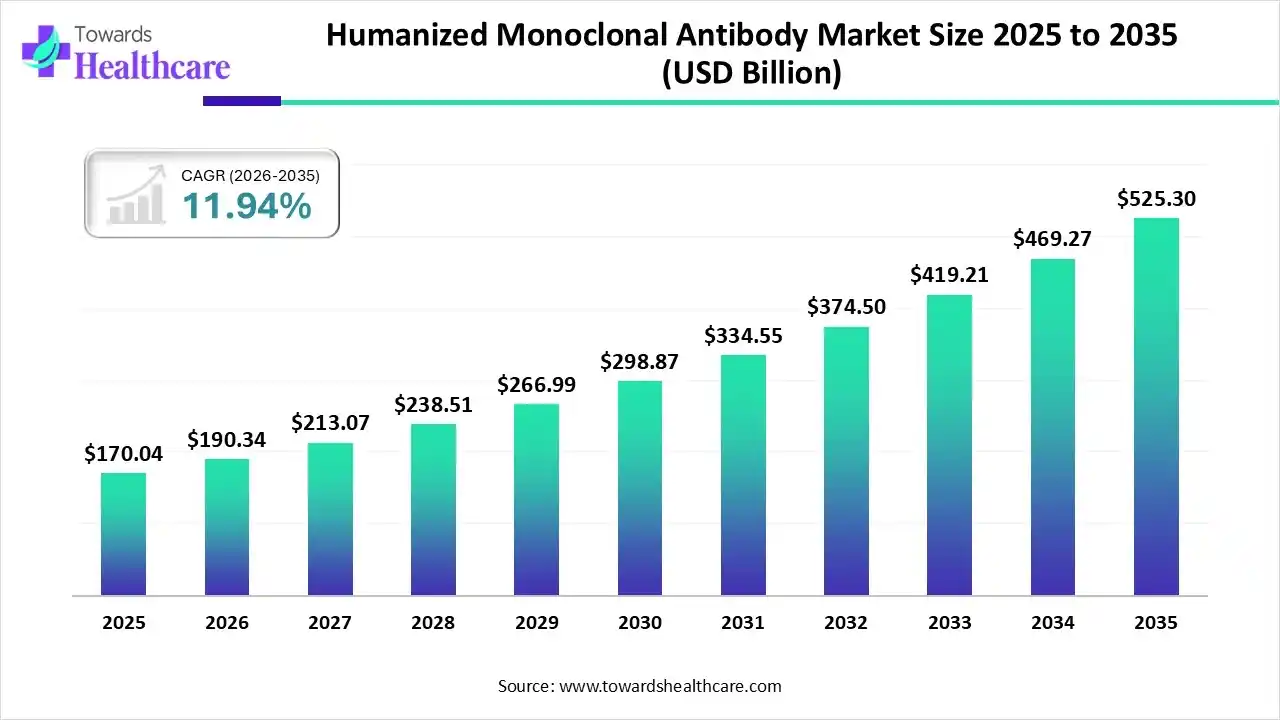
The global humanized monoclonal antibody market size was estimated at USD 170.04 billion in 2025 and is predicted to increase from USD 190.34 billion in 2026 to approximately USD 525.3 billion by 2035, expanding at a CAGR of 11.94% from 2026 to 2035.
The humanized monoclonal antibody market is increasing as this antibody has several promising therapeutic applications in the management of asthma, autoimmune diseases, cancer, poisoning, sepsis, substance abuse, viral infections, and various types of diseases.
The humanized monoclonal antibody market is increasing because these therapies are creating exciting novel opportunities in areas such as autoimmune disorders, oncology, infectious diseases, and beyond. Fully human monoclonal antibodies (mAbs) are certainly leading the way in this growth. They provides advance tolerability, lower adverse effects, and greater efficiency for a variety of diseases. Recent advancements in technologies such as transgenic mice and phage display platforms efficiently create these human-derived antibodies, which have massive specificity. Human mAbs are generally crafted with structures that support them in connecting with their targets more precisely and with great strength.
For Instance,
Integration of AI-driven technology in humanized monoclonal antibody drives the growth of the market, as AI-based technology analyze massive number of datasets of antibody structures and their communications with antigens, learning patterns that allow it to envisage which antibody sequences will be both efficacious and negligibly immunogenic in humans. It rapidly screens and optimizes antibody applicants, significantly lowering the time and expenses related to traditional processes. This incorporation of AI-driven approaches in antibody engineering shows a transformative leap forward in making safer and more efficient antibody-based therapies. AI-based technology, predominantly machine learning and deep learning algorithms, has altered antibody humanization by presenting a more systematic and data-driven approach.
Bispecific antibodies are allows to recruit immune cells into the vicinity of tumours and enable them to thrive in the immunosuppressive cancer microenvironment, owing to their capability to overcome the properties of different inhibitory factors and offer stimulatory signals able to restore antitumor actions.
Antibody-drug conjugates (ADCs) are novel biopharmaceutical products in which a monoclonal antibody is connected to a small molecule drug with a stable linker. Most of the ADCs developed so far are for treating oncology, but there is massive potential for using ADCs to manage other diseases.
Fully human mAbs have an inferior immunogenicity, which increases both safety and efficiency in therapy. This makes them particularly helpful in managing cancer, autoimmune diseases, and infections.
| Key Elements | Scope |
| Market Size in 2026 | USD 190.34 Billion |
| Projected Market Size in 2035 | USD 525.3 Billion |
| CAGR (2026 - 2035) | 11.94% |
| Leading Region | North America by 46.5% |
| Market Segmentation | By Indication, By Application, By End-User, Regional Outlook |
| Top Key Players | F. Hoffmann-La Roche Ltd, AbbVie Inc., Amgen Inc., Johnson & Johnson, Bristol Myers Squibb, Merck & Co., Inc. |
Which Indication Led the Humanized Monoclonal Antibody Market in 2025?
In 2025, the oncology segment held the dominant market share with approximately 48.5% share in 2025, as monoclonal antibodies (mAbs) transformed cancer management by offering extremely targeted and efficient therapies that precisely attack cancer cells. Monoclonal antibodies (mAbs) have revolutionized cancer management by offering highly targeted and efficient therapies that precisely attack cancer cells, thus lowering the likelihood of adverse events (AEs) in patients.
Autoimmune Diseases
Whereas the autoimmune diseases segment is the fastest-growing in the market, as monoclonal antibodies (mAbs) are revolutionizing how autoimmune diseases are managed. Unlike wide immunosuppressants, mAbs target specific immune pathways, supporting control of symptoms while lowering chronic tissue damage. Monoclonal antibodies supports immune system in fighting diseases or blocking proteins that cause diseases. Monoclonal antibodies (mAbs) are being applied to detect, prevent, and treat a wide spectrum of malignancies and infectious and autoimmune diseases.
Why did the Clinical Drugs Segment Dominate the Market in 2025?
The clinical drugs segment is dominant and fastest-growing in tissue regenerative therapy, with approximately 82% share in 2025, as humanized antibodies have improved stability in the human body. Human antibodies are certainly helped by the immune system, enabling them to circulate for lengthy periods. Humanized antibodies interrelate more effectively with human immune trails because of their naturally compatible structure. Humanized antibodies have redesigned the biologics landscape by enhancing both safety and therapeutic performance.
Laboratory Research
Whereas the laboratory research segment is the fastest-growing in the market, as mAbs have their high selectivity, specificities, and affinities which are optimized towards closely biomolecule of interest. Furthermore, their recombinant engineering and manufacturing in mammalian cell lines exploits their batch-to-batch quality, purity, and immunologic compatibility with patients. They provide better tolerability, reduced adverse effects, and advanced effectiveness for a variety of diseases.
Why did the Hospitals Segment Dominate the Market in 2025?
The hospitals segment is dominant in the humanized monoclonal antibody market with approximately 42% share in 2025, as emerging human monoclonal antibodies allows to effectively manipulate their effector functions while evading immunogenicity seen with rodent antibodies. Humanization of monoclonal antibodies is beneficial as it reduces the chances of provoking an immune response in patients. Antibody humanization improves the efficacy of therapeutic antibodies derived from non-human sources.
Specialty & Outpatient Centers
Whereas the specialty & outpatient centers segment is the fastest-growing in the market, as production of human monoclonal antibodies allows to efficiently manipulate their effector functions while evading immunogenicity seen with rodent antibodies. Antibody immunogenicity outcomes from the degree to which the host immune system recognizes and reacts to these medicinal agents in speciality and outpatient centers.
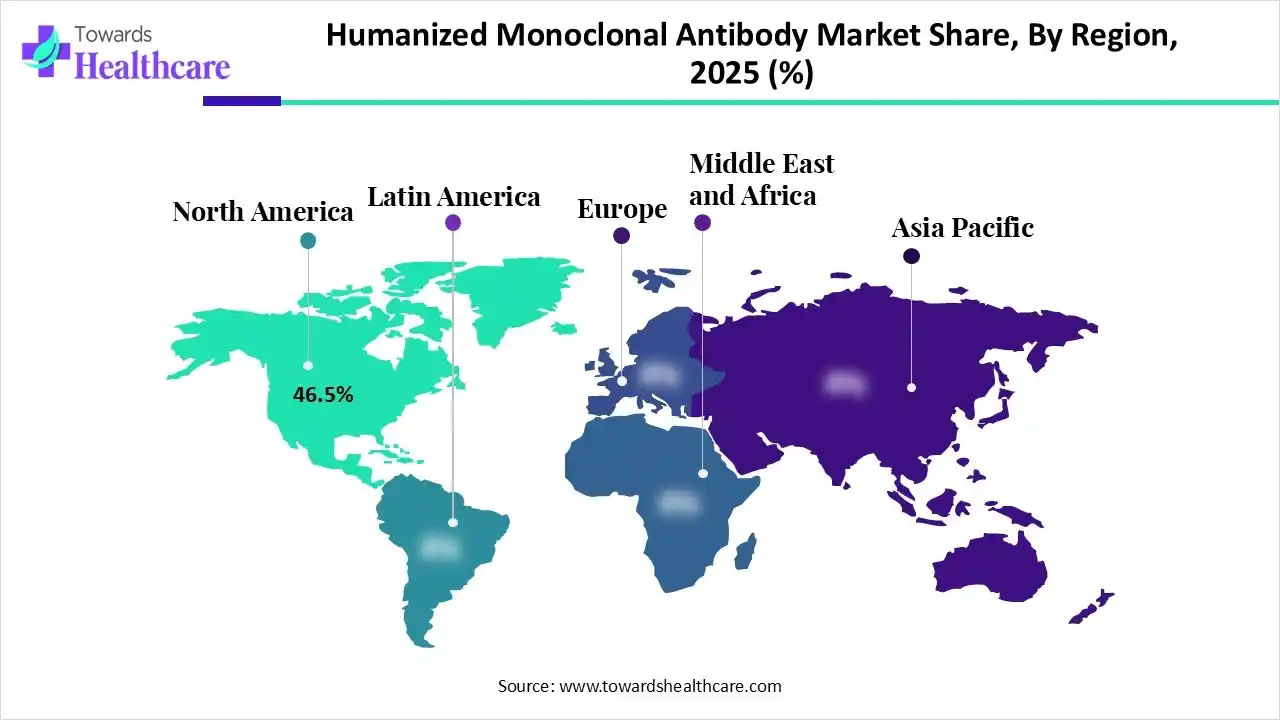
In 2025, North America dominated the humanized monoclonal antibody market with approximately 46.5% share, as this region is the world’s main foreign investor and the largest recipient of foreign direct investments, almost each year for numerous decades. Major companies have frequently been using available expedited pathways like priority review, accelerated approval, and breakthrough therapy designation. North America leads in the number of active clinical trials, particularly in oncology and autoimmune diseases, where humanized antibodies are the preferred action due to their lower immunogenicity compared to older chimeric types.
For Instance,
U.S. Market Trends
The United States has long led the world in oncology research. It has invested more in oncology research than other countries, including more than US$7.2 billion yearly via the National Cancer Institute, which increases the growth of the market. The US government invests significantly in health research and development (R&D), as well as analysis of the health outcomes. Novelty by the healthcare industry is vital for manufacturing breakthrough products that advance healthcare.
Asia Pacific is expected to see rapid growth in the humanized monoclonal antibody market, driven by growing CVD epidemics in this region, including demographic shifts, socioeconomics, living environments, and lifestyles, which are increasing the demand for humanized monoclonal antibodies. The increase in digital health services and rising research in clinical trials highlight India's role as a home for innovative healthcare research.
India Market Trends
India is set to become a worldwide biopharmaceutical manufacturing hub. The increasing digital health services and spending in clinical trials highlight India's potential for innovative medical research. Indian regulations offer various incentives and help for the biotech sector, including tax advantages, subsidies, and funding for research and development.
Europe is significantly growing in the humanized monoclonal antibody market, due to partnering with local companies, research organizations, and regulatory bodies. Spending in research and development in India enables foreign businesses to leverage local novelties and adapt products. A well-established, progressive biotechnology field with important big pharma investments and manufacturing abilities.

| Company | Headquarters | Latest Update |
| F. Hoffmann-La Roche Ltd | Switzerland | In December 2025, Roche announced that the U.S. Food and Drug Administration (FDA) had approved additional indications for its PATHWAY anti-HER2/neu (4B5) Rabbit Monoclonal Primary Antibody and VENTANA HER2 Dual ISH DNA Probe Cocktail tests. |
| AbbVie Inc. | United States | In October 2025, AbbVie announced it would unveil novel data from its vigorous antibody-drug conjugate (ADC) platform at the 2025 European Society for Medical Oncology (ESMO) congress. |
| Amgen Inc. | United States | In April 2025, Amgen announced that the U.S. Food and Drug Administration (FDA) had approved UPLIZNA as the first and only treatment for adults living with Immunoglobulin G4-related disease (IgG4-RD). |
| Johnson & Johnson | United States | In April 2025, Johnson & Johnson announced that the U.S. Food and Drug Administration (FDA) had approved IMAAVY, a human FcRn-blocking monoclonal antibody, for the management of generalized myasthenia gravis (gMG). |
| Bristol Myers Squibb | United States | In December 2025, Harbour BioMed announced a multi-year, global strategic partnership and license agreement with Bristol Myers Squibb to discover and develop next-generation many-specific antibodies. |
| Merck & Co., Inc. | United States | In October 2025, Merck announced it had initiated three Phase 2b trials evaluating the safety and efficacy of tulisokibart, an investigational humanized monoclonal antibody targeting tumor necrosis factor (TNF)-driven cytokine 1A (TL1A). |
By Indication
By Application
By End-User
Regional Outlook
February 2026
January 2026
November 2025
October 2025