February 2026

The global sickle cell anemia testing and screening market size was estimated at USD 482.2 million in 2025 and is predicted to increase from USD 540.74 million in 2026 to approximately USD 1516.47 million by 2035, expanding at a CAGR of 12.14% from 2026 to 2035.
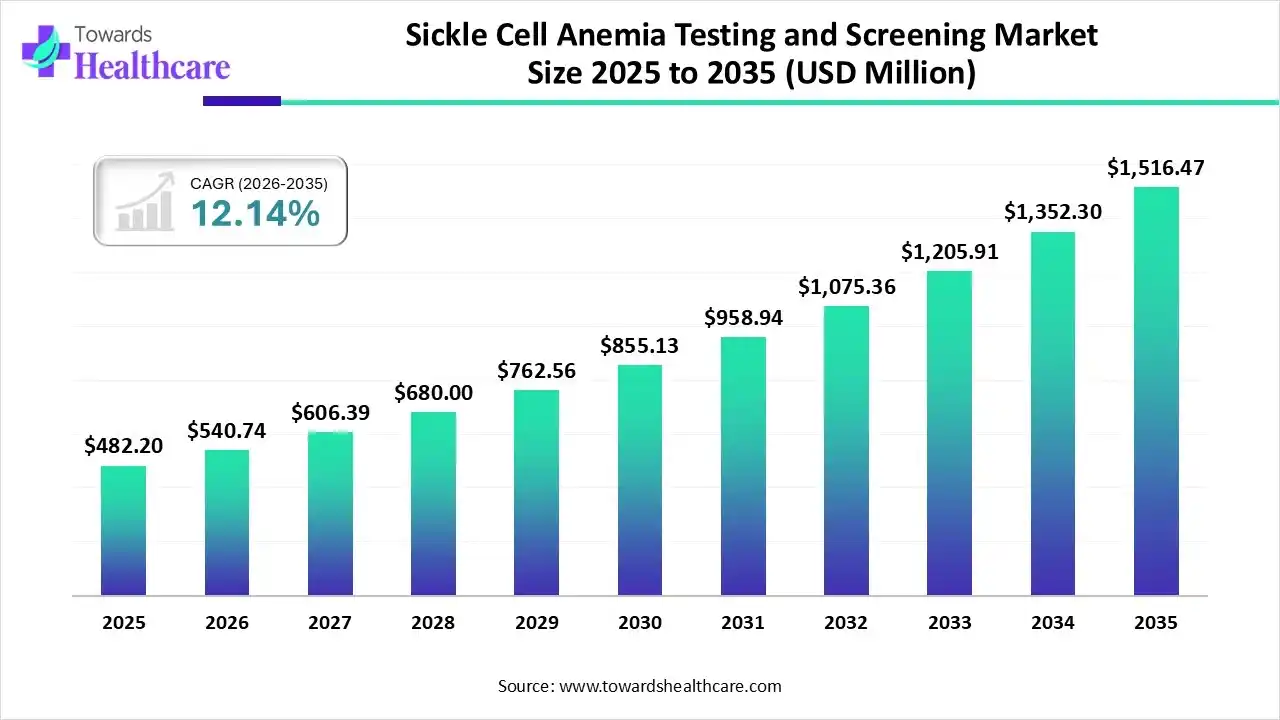
The sickle cell anemia testing and screening market is expanding rapidly worldwide due to rising disease prevalence, newborn screening initiatives, and technological advances like genetic and point-of-care diagnostics. Demand is growing in both developed and emerging regions, with efforts to improve early detection and access to accurate, rapid testing driving broader adoption and infrastructure investment.
| Key Elements | Scope |
| Market Size in 2026 | USD 540.74 Million |
| Projected Market Size in 2035 | USD 1516.47 Million |
| CAGR (2026 - 2035) | 12.14% |
| Leading Region | North America |
| Market Segmentation | By Technology, By Age Group, By Sector Type, By Region |
| Top Key Players | Request A Test, Ltd, HALCYON BIOMEDICAL INCORPORATED, HemoType, Silver Lake Research Corporation, Hemex Health, Bio-Rad Laboratories, Inc. |
The sickle cell anemia testing and screening market is growing largely independent of AI due to mandatory newborn screening programs, rising disease prevalence, and strong government-led public health initiatives. Growth is driven by established diagnostic methods, expanding healthcare infrastructure, and increased awareness of early detection benefits, rather than reliance on advanced AI-based technologies in routine screening workflows.
Governments and health agencies are broadening mandatory screening initiatives worldwide, increasing early diagnosis rates and reducing complications. This trend will enhance public health outcomes and drive sustained demand for reliable testing solutions.
Innovations like rapid assays and more sensitive genetic tests are improving accuracy and turnaround times. Future adoption of these technologies will streamline screening processes and widen accessibility.
Improved healthcare infrastructure and awareness in Africa, Asia, and Latin America are boosting market growth. Continued investment and support in these regions will expand screening coverage and patient access in the coming years.
Why Did the Hemoglobin Electrophoresis Segment Dominate in the Market in 2025?
The hemoglobin electrophoresis segment held the largest sickle cell anemia testing and screening market share in 2025, due to its high diagnostic accuracy, reliability, and widespread clinical acceptance for identifying abnormal hemoglobin variants. Its cost-effectiveness, standardized protocols, and routine use in hospitals and reference laboratories for confirmatory testing supported strong adoption across both developed and developing healthcare systems.
Point-of-care Tests
The point-of-care tests segment is expected to grow at the fastest CAGR during the forecast period due to increasing demand for rapid, affordable, and easy-to-use diagnostic solutions. These tests support early detection, especially in newborn and community-based screening programs. Their minimal infrastructure requirement quick turnaround times, and suitability for rural and resource-limited settings are accelerating adoption across emerging and developed healthcare markets.
What made the Newborn Screening (12 months and below) Segment Dominant in the Market in 2025?
The newborn screening (12 months and below) segment led the sickle cell anemia testing and screening market due to mandatory screening policies in many countries and the critical need for early diagnosis of sickle cell anemia. Early detection enables timely interventions, reduces infant mortality, and prevents severe complications. Strong government support, integration into routine neonatal care, and growing awareness among healthcare providers and parents further drove widespread adoption of newborn screening programs.
Other age groups (1 to 25 & above 60 years)
The other age groups (1 to 25 & above 60 years) segment is expected to grow at the fastest CAGR due to increasing diagnosis of undetected cases in adolescents and adults. Rising awareness, expanded carrier screening, and improved access to healthcare services are driving testing beyond newborns. Additionally, genetic counselling needs, premarital screening initiatives, and better disease management programs are boosting demand for testing in this age group.
How Does the Government Labs Segment Dominate the Market in 2025?
The government labs segment dominated the sickle cell anemia testing and screening market due to strong public funding and extensive healthcare infrastructure. These labs support large-scale and diagnostic programs under national health initiatives. With standardized protocols, trained staff, and high patient volumes, they provide reliable and affordable testing. Their role in early disease detection and preventive care ensures widespread accessibility, reinforcing their leading position in the market.
Private Labs
The private labs segment is expected to grow at the fastest CAGR due to increasing demand for personalized, convenient, and rapid diagnostic services. Rising awareness of early disease detection, advanced technologies, and high-quality testing options drives patient preference for private facilities. Flexible service offerings, shorter turnaround times, and collaborations with healthcare providers further boost their adoption, positioning private labs for significant market growth during the forecast period.
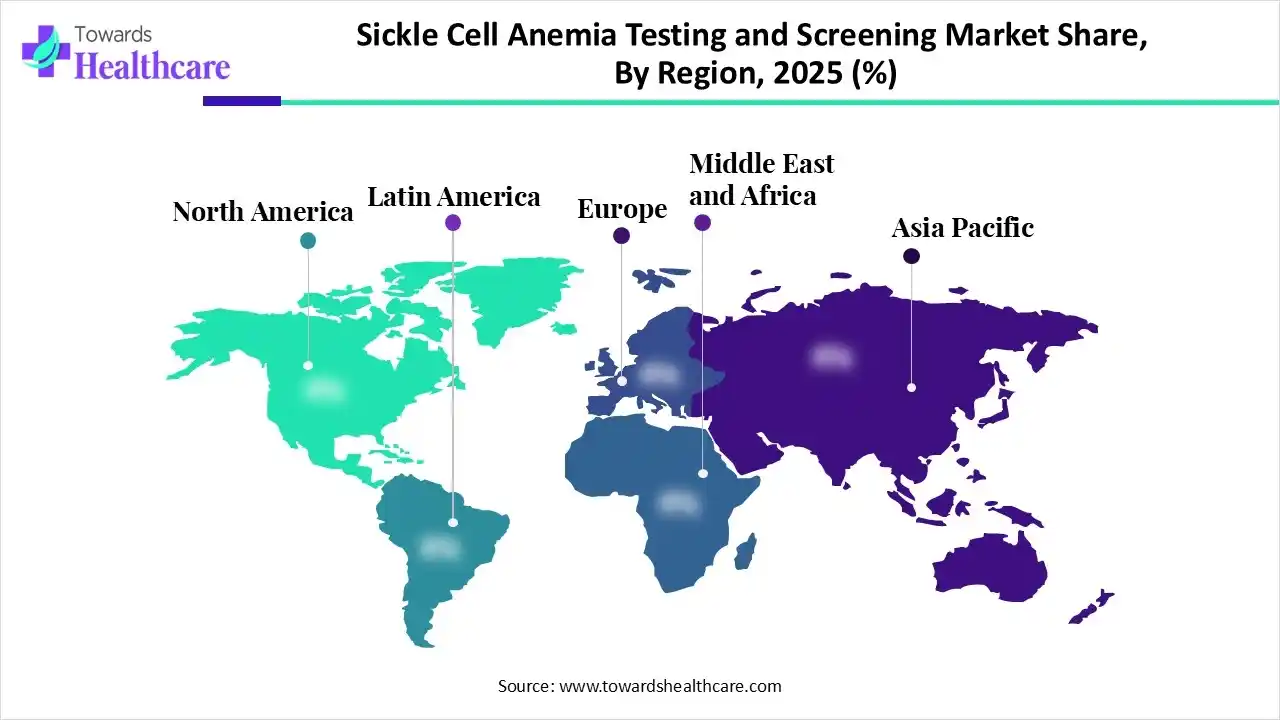
North America dominated the sickle cell anemia testing and screening market in 2025 due to advanced healthcare infrastructure, widespread newborn screening programs, and high awareness of early diagnosis. Strong government support, well-established reimbursement policies, and availability of advanced diagnostic technologies further fueled market growth. Additionally, significant investments by private and public sectors in research and development, along with growing patient awareness, contributed to the region’s leading position.
U.S. Leads Market with Innovation and Growing Awareness
The U.S. led the sickle cell anemia testing and screening market in 2025 by generating the highest revenue, driven by advanced healthcare infrastructure, widespread newborn screening, and high patient awareness. Strong government support, established reimbursement policies, and the presence of key players offering innovative diagnostic solutions further strengthened market growth, securing the country’s top position.
Asia Pacific is expected to grow at the fastest CAGR in the sickle cell anemia testing and screening market due to increasing awareness of sickle cell anemia, rising healthcare investments, and expanding diagnostic infrastructure. Growing government initiatives, adoption of advanced screening technologies, and a large patient population drive demand. Additionally, the presence of emerging private labs and rising early detection programs further accelerates market growth in the region.
India Leads the Race in Sickle Cell Testing with Rising Awareness and Improved Diagnostics
India is expected to grow at the fastest CAGR due to increasing awareness of sickle cell anemia, expanding healthcare infrastructure, and government-led screening programs. Rising demand for early diagnosis, adoption of advanced diagnostic technologies, and growth of private laboratories further boost the market. A large patient population and focused public health initiatives accelerate growth during the forecast period.
Europe is expected to grow at a notable CAGR in the sickle cell anemia testing and screening market due to increasing government initiatives, well-established healthcare infrastructure, and rising awareness of sickle cell anemia. Adoption of advanced diagnostic technologies, availability of specialized private and public laboratories, and growing investments in early screening programs contribute to market growth. Supportive reimbursement policies further enhance regional adoption during the forecast period.
UK Sees Rapid Uptake in Sickle Cell Testing Driven by Decentralized Diagnostics
The UK is expected to witness rapid sickle cell anemia testing and screening market growth due to increasing demand for decentralized and point-of-care testing, improved access to genetic diagnostics, and rising focus on community-based healthcare delivery. Growing investments in laboratory automation, digital health integration, and enhanced referral pathways for high-risk populations further support faster market expansion during the forecast period.
R&D
Clinical Trials
Patient Support and Services

| Companies | Headquarters | Offerings |
| Request A Test, Ltd | California, USA | Provides affordable sickle cell tests with fast turnaround and online results through a partnership with CLIA-certified labs. |
| HALCYON BIOMEDICAL INCORPORATED | USA | Works on advanced blood testing and diagnostic technologies that support clinical labs, with potential contributions to hematology diagnostics, including markets related to hemoglobin disorders. |
| HemoType | USA | HemoTypeSC rapid diagnostic test for hemoglobin variants used in newborn and field screening for sickle cell disease and carriers. |
| Silver Lake Research Corporation | USA | Developer of HemoType SC, a rapid point-of-care lateral-flow test that detects multiple hemoglobin variants for sickle cell disease and trait screening. |
| Hemex Health | Colorado, USA | Gazelle point-of-care diagnostic platform that provides affordable, easy-to-use testing to identify and quality haemoglobin disorders, including sickle cell disease and beta-thalassemia, with results in minutes and cloud connectivity options. |
| Bio-Rad Laboratories, Inc. | California, USA | Offers advanced haemoglobin testing systems widely used for precise detection and monitoring of sickle cell disease and other haemoglobinopathies in clinical labs. |
By Technology
By Age Group
By Sector Type
By Region
February 2026
February 2026
February 2026
February 2026
