February 2026

The parenteral antibiotics market size was estimated at USD 25.3 billion in 2025 and is predicted to increase from USD 26.4 billion in 2026 to approximately USD 38.7 billion by 2035, expanding at a CAGR of 4.34% from 2026 to 2035.
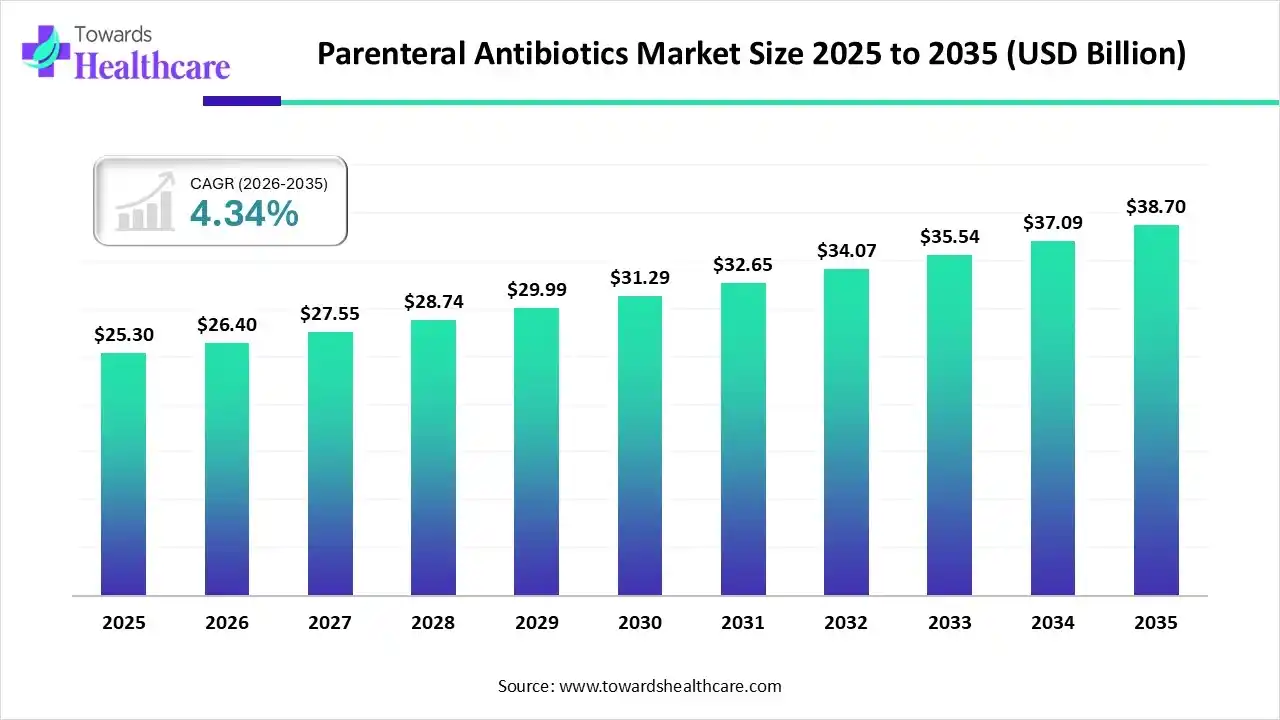
The parenteral antibiotics market is gaining momentum globally, followed by the government of India’s national treatment guidelines for antimicrobial use in infectious disease syndromes. Moreover, the global market is driven by the approvals for novel combination therapies for multidrug-resistant (MDR) infections.
| Key Elements | Scope |
| Market Size in 2026 | USD 26.4 Billion |
| Projected Market Size in 2035 | USD 38.7 Billion |
| CAGR (2026 - 2035) | 11.94% |
| Leading Region | North America by 36% |
| Market Segmentation | By Drug Class, By Route of Administration, By Indication/Infection Type, By End-User, By Drug Type, By Region |
| Top Key Players | Pfizer Inc., GSK Plc, Merck & Co., Inc., Sandoz Group AG, AbbVie Inc., Bayer AG, Cipla, Sun Pharmaceutical Industries Ltd., Dr. Reddy’s Laboratories, Aurobindo Pharma |
The global parenteral antibiotics market comprises injectable antibacterial drugs administered via intravenous (IV), intramuscular (IM), or subcutaneous routes for the treatment of moderate to severe bacterial infections. These therapies are critical in hospital and acute-care settings where rapid onset of action, high bioavailability, and precise dosing are required. The market includes branded and generic formulations across major antibiotic classes used to manage sepsis, hospital-acquired infections (HAIs), community-acquired infections, and multidrug-resistant (MDR) pathogens. Growth is driven by rising hospitalization rates, increasing prevalence of antimicrobial resistance (AMR), expanding surgical volumes, and continued demand for empiric and targeted IV antibiotic therapy in critical care.
AI is vital in parenteral antibiotic manufacturing to enhance sterility, safety, and regulatory compliance. AI is transforming pharmaceutical manufacturing and the market by improving precision, efficiency, and quality. AI-driven technologies like computer vision, machine learning, and predictive analytics improve process control in filling, formulation, sealing, and packaging. These AI tools ensure compliance with Good Manufacturing Practices (GMP).
How does the Beta-Lactams Segment Dominate the Parenteral Antibiotics Market in 2025?
The beta-lactams segment dominated the market in 2025, with a revenue share of approximately 45%, owing to the major role of beta-lactams in parenteral therapy, including direct bactericidal activity and treatment of severe and multi-drug resistant infections. Beta-lactams like ceftriaxone and ertapenem are widely used in outpatient parenteral antibiotic therapy. They are ideally used in outpatient parenteral antibiotic therapy for long-term treatment of bone infections.
Lipopeptides
The lipopeptides segment is expected to grow at the fastest CAGR of approximately 8-10% in the parenteral antibiotics market during the forecast period due to the wide clinical use of major lipopeptides such as Daptomycin, Polymyxin B, and Colistin. They are in high demand due to the limitations of conventional therapies. They have a unique ability to target and disrupt bacterial cell membranes.
What made Intravenous the Dominant Segment in the Parenteral Antibiotics Market in 2025?
The intravenous segment dominated the market in 2025, with a revenue share of approximately 75%, owing to the important clinical applications and rapid therapeutic effect of the intravenous route for parenteral antibiotics. It offers immediate bioavailability and manages severe infections. It is ideal for medical emergencies such as sepsis for a rapid therapeutic effect of antibiotics on tissues.
Subcutaneous
The subcutaneous segment is estimated to grow at the fastest CAGR of approximately 7-9% in the parenteral antibiotics market during the predicted timeframe due to its critical role as an alternative to intravenous or intramuscular administration. This route is ideal in palliative, geriatric, and outpatient care. The potential advantages of this route are the ease of use and vein preservation.
How did the Hospital-Acquired Infections Segment Dominate the Parenteral Antibiotics Market in 2025?
The hospital-acquired infections segment dominated the market in 2025, with a revenue share of approximately 30%, owing to the intensity, selection, and duration of treatment. The rising prevalence of multidrug-resistant (MDR) pathogens in clinical settings drives segmental growth. The surgical site infections are treated based on the wound type.
Sepsis & Septic Shock
The sepsis & septic shock segment is anticipated to grow at a notable CAGR of approximately 10-12% in the parenteral antibiotics market during the upcoming period due to management considerations such as altered pharmacokinetics, prolonged infusions, and de-escalation. The specific health-related history of patients leads to the addition of specialized parenteral agents like vancomycin. The specific life-threatening conditions and medical emergencies directly impact survival rates.
Which Segment by End-User Dominated the Parenteral Antibiotics Market in 2025?
The hospitals segment dominated the market in 2025, with a revenue share of approximately 68%, owing to the huge adoption of parenteral antibiotics in hospitals for the initial treatment of severe infections, surgical prophylaxis, and the management of non-absorbable cases. These antibiotics deliver cost efficiency and improved patient outcomes. The commonly used antibiotics in hospitals are penicillins, cephalosporins, aminoglycosides, and lipopeptides.
Ambulatory Infusion Centers
The ambulatory infusion centers segment is predicted to grow at a rapid rate with a CAGR of approximately 10-12% in the parenteral antibiotics market during the studied period due to the importance of these centers as a bridge between hospital care and home-based self-administration. The infusion centers help with direct administration, rapid infusion protocols, and admission avoidance. They are empowered with infectious disease specialists, infusion nurses, and ID-trained pharmacists.
Why did the Generic Parenteral Antibiotics Segment dominate the Parenteral Antibiotics Market in 2025?
The generic parenteral antibiotics segment dominated the market in 2025, with a revenue share of approximately 62%, owing to their assistance in managing severe and critical infections. The expansion of global access to essential medicines drives the demand for generic parenteral antibiotics. The public health policies and outpatient therapies ensure affordable access to parenteral antibiotics.
Branded Parenteral Antibiotics
The branded parenteral antibiotics segment is predicted to grow at a rapid rate with a CAGR of approximately 8-10% in the parenteral antibiotics market during the studied period due to their role in the clinical management of severe infections and overcoming antimicrobial resistance. They enable outpatient care with the help of long-acting formulations and the ease of dosing. They are used in critical care and as primary therapy for sepsis.
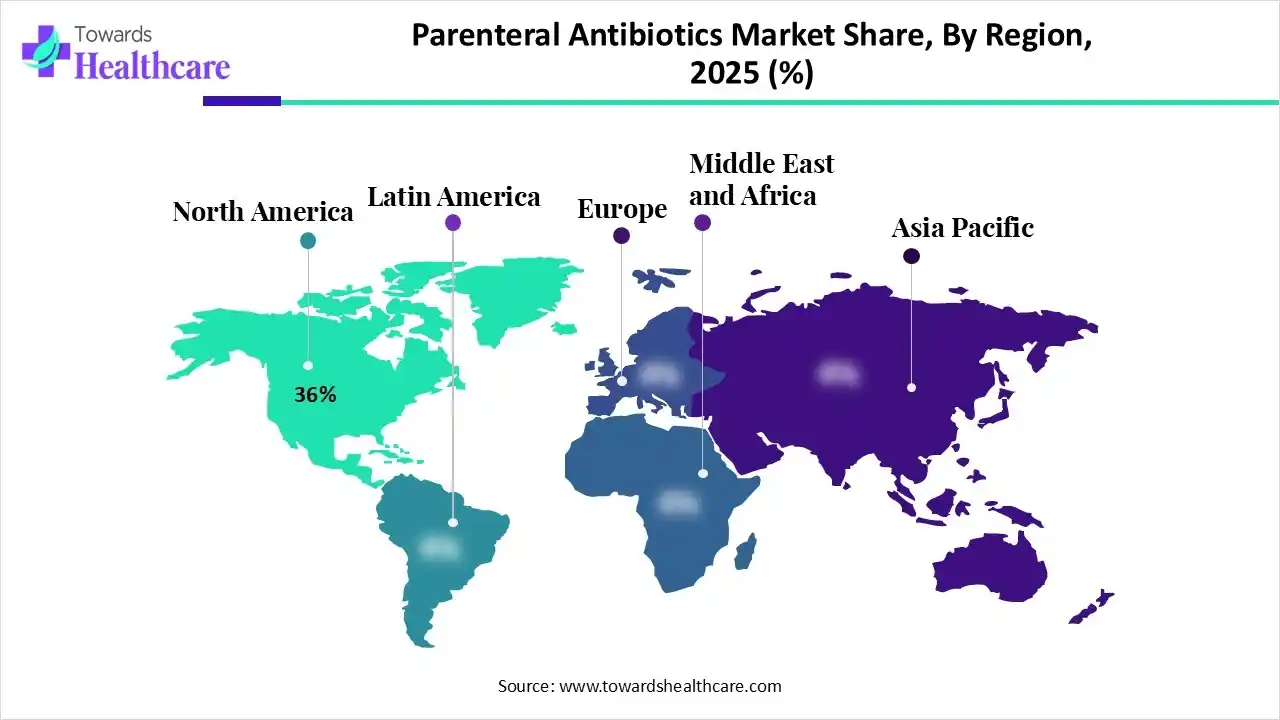
North America dominated the market in 2025, with a revenue share of approximately 36%, owing to the rising incidence of severe infections, surgical and medical procedures, and government incentives. The North American government initiatives focused on securing the supply of parenteral antibiotics, addressing antimicrobial resistance, and tracking product development. Some of the major actions taken by the North American countries, like Canada, are the antimicrobial economic incentives pilot project and the target zero campaign. Moreover, Mexico has taken steps to expand domestic pharmaceutical production and plants for injectable medicines.
U.S. Market Trends
The parenteral antibiotics market in the U.S. is driven by strategic product launches, high clinical efficacy, and the expansion of surgical procedures. The Center for Drug Evaluation and Research (CDER) of the U.S. FDA approved several drugs, including generic drugs for treating various indications.
In February 2025, the U.S. FDA approved the antibiotic named EMBLAVEO for treating adults who are dealing with complex intra-abdominal infections with limited or no treatment options.
Asia Pacific is expected to grow at the fastest CAGR of approximately 10-12% in the market during the forecast period due to increasing healthcare expenditure, new product approvals, and growth in hospital-acquired infections. The World Health Organization (WHO), along with the South-East Asia and Western Pacific regions, moved forward to tackle antimicrobial resistance. This initiative was led by the government of Japan and supported by almost 30 Asian Pacific countries, such as Cambodia, the Democratic People’s Republic of Korea, Indonesia, Japan, Malaysia, the Maldives, India, etc.
India Market Trends
The Indian parenteral antibiotics market is accelerated by the national action plan on antimicrobial resistance by the government of India and the increased production of novel antibiotics. In December 2024, the Ministry of Chemicals and Fertilizers reported that Nafithromycin is India’s first indigenous Macrolide antibiotic, which highlights the country’s potential in pharmaceutical innovation.
Europe is expected to grow at a notable rate in the market in 2025, driven by a strong focus on advanced research and clinical trials. The European Commission remains ahead in making partnerships with other government entities for research and innovation in antimicrobial resistance. This further highlights its €253 million partnership on the One Health AMR project to advance R&D.
In December 2025, the World Health Organization (WHO) and the Health Emergency Preparedness Authority (HERA) of the European Commission made a partnership through a €3.5 million agreement to fight against antimicrobial resistance under the EU4Health programme.
Germany Market Trends
The parenteral antibiotics market in Germany is significantly growing through the country’s national pharma strategy and its strong support to contract research organizations (CROs) and sponsors to advance R&D and innovation. Germany introduced the Medical Research Act, and the country supports initiatives for combating antimicrobial resistance.

| Sr. No. | Name of the Company | Headquarter | Latest Update |
| 1 | Pfizer Inc. | New York, U.S. | In March 2024, Pfizer Inc. received a positive CHMP opinion for its novel antibiotic named EMBLAVEO. |
| 2 | GSK Plc | London, UK | In November 2025, GSK and Fleming Initiative scientists united to target AMR with advanced AI. |
| 3 | Merck & Co., Inc. | Rahway, New Jersey, USA | In December 2025, Merck received a positive CHMP opinion for WINREVAIR. |
| 4 | Sandoz Group AG | Basel, Switzerland | In March 2024, Sandoz Group AG opened a new antibiotic production facility in Austria. |
| 5 | AbbVie Inc. | North Chicago, Illinois | In July 2025, AbbVie Inc. and Pfizer planned to advance a pediatric antibiotic study with ATM-AVI. |
| 6 | Bayer AG | Leverkusen, Germany | In November 2025, the WHO and Bayer AG collaborated to eliminate three tropical diseases. |
| 7 | Cipla | Mumbai | In May 2025, Cipla launched ZEMDRI in India to target AMR. |
| 8 | Sun Pharmaceutical Industries Ltd. | Mumbai | In November 2025, Sun Pharmaceutical Industries Ltd reported that its U.S. innovative drug sales surpassed generics. |
| 9 | Dr. Reddy’s Laboratories | Hyderabad, Telangana, India | In May 2024, Dr. Reddy’s Laboratories launched broad-spectrum antibiotics in the USA. |
| 10 | Aurobindo Pharma | Hyderabad | In October 2024, Aurobindo Pharma received the U.S. FDA approval for an antibiotic tablet. |
By Drug Class
By Route of Administration
By Indication/Infection Type
By End-User
By Drug Type
By Region
February 2026
February 2026
February 2026
February 2026