March 2026

The global acute respiratory distress syndrome market size was estimated at USD 6.29 billion in 2025 and is predicted to increase from USD 6.71 billion in 2026 to approximately USD 11.98 billion by 2035, expanding at a CAGR of 6.65% from 2026 to 2035.
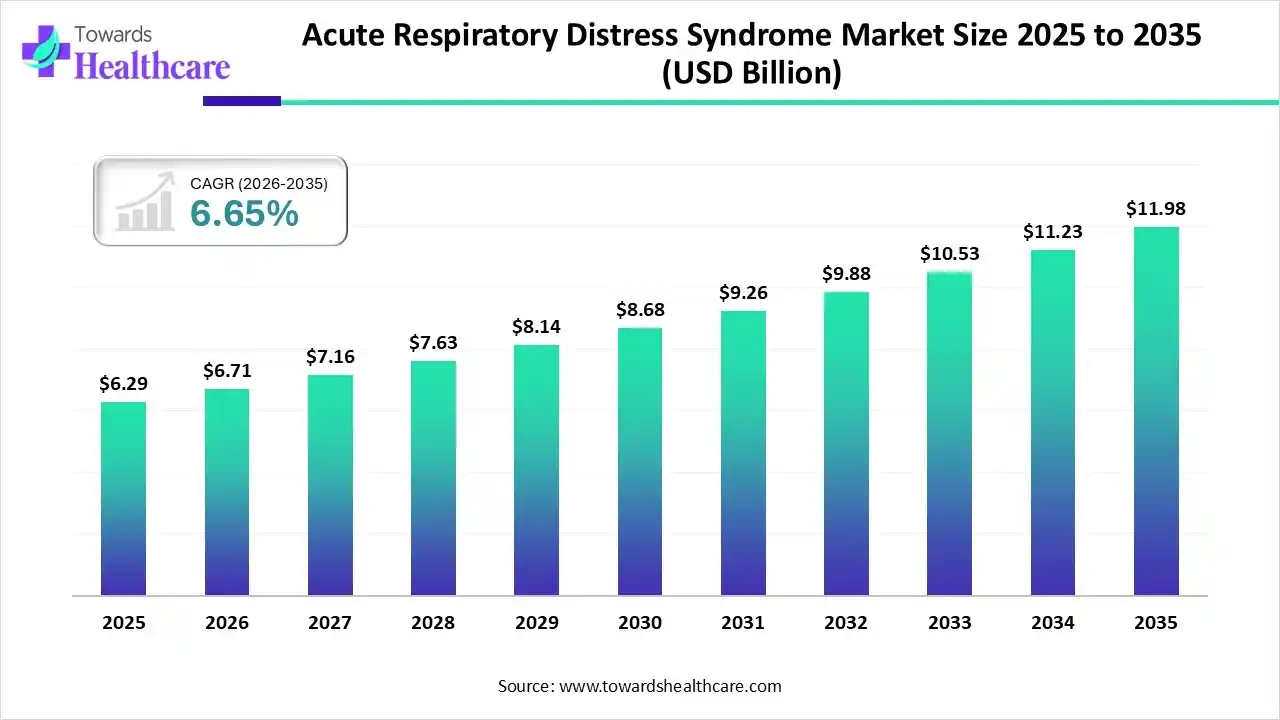
The acute respiratory distress syndrome market is growing due to an increasing aging population, which is more prone to severe lung diseases, and the rising burden of lower respiratory infections (LRIs) remains the global leading infectious cause of death.
| Key Elements | Scope |
| Market Size in 2026 | USD 6.71 Billion |
| Projected Market Size in 2035 | USD 11.98 Billion |
| CAGR (2026 - 2035) | 6.65% |
| Leading Region | North America |
| Market Segmentation | By Drug Class, By Route of Administration, By Distribution Channel, Regional Outlook |
| Top Key Players | Athersys Inc., mondoBIOTECH AG, HEALIOS K.K., GlaxoSmithKline plc., Pfizer Inc., Teva Pharmaceuticals USA Inc., Altor BioScience, Faron Pharmaceuticals, Ltd. |
The integration of AI-driven technology into the acute respiratory distress syndrome drives the growth of the market, as AI-driven technology is used in diagnosis, risk management, prediction of severity and mortality, management, and decision making for physicians in ARDS. AI-driven technology incessantly monitors patient information, involving respiratory patterns, lung mechanics, and blood gas levels. AI algorithms regulate ventilation parameters in real time, confirming optimum support for the patient's respiratory system.
Which Drug Class Led the Acute Respiratory Distress Syndrome Market in 2025?
In 2025, the bronchodilators segment held the dominant market as this drug supports relieving symptoms of asthma, acute respiratory distress syndrome, and other lung diseases by relaxing the muscles around patients' airways and also helps in clearing mucus from the lungs. Bronchodilator therapy often lowers symptoms of air-flow obstruction by calming airway smooth muscle (bronchodilation), reducing dyspnoea, and enhancing quality of life.
Steroids and Antibiotics
Whereas the steroids and antibiotics segment is the fastest-growing in the market, as steroids decrease the mortality rate in patients with acute respiratory distress syndrome (ARDS) via an anti-inflammatory effect. Steroids have both anti-inflammatory and anti-fibrotic characteristics; they still show to be the standard-studied pharmacologic intervention for late ARDS. Corticosteroids in ARDS have shown promise as a pharmacotherapeutic by improving the coordinated resolution of ARDS.
Why did the Oral Segment Dominate the Market in 2025?
The oral segment is dominant in the acute respiratory distress syndrome market in 2025, as oral route administration of drugs is a convenient, affordable, and widely used medication administration route. The significant site of drug absorption is generally the small intestine, and the bioavailability of the medicine is influenced by the quantity of drug absorbed in the intestinal epithelium. It is the humblest, most suitable, and safest route of drug administration.
Inhalation
Whereas the inhalation segment is the fastest growing in the market, as the inhaled route delivers the medicine directly to the lungs, the dose needed is smaller than when given by mouth, and side effects are reduced. Inhalation delivery enables a much higher concentration of the drug to reach the lungs, causing to rapid and more efficient treatment. Inhaled medications enhance exercise tolerance, energy levels, and sleep quality, and lower the challenges of other respiratory infections.
Why did the Hospital Pharmacies Segment Dominate the Market in 2025?
The hospital pharmacies segment is dominant in the acute respiratory distress syndrome market in 2025, as this type of pharmacy has resources that dramatically aid a hospital's revenue cycle from the point of service to claim submission. The hospital pharmacist is allowed to purchase, store, handle, price, and dispense medications.
Online Pharmacies
Whereas the online pharmacies segment is the fastest growing in the market, as this pharmacy integrates payment, billing, and insurance claims. So transactions occur rapidly and precisely with improved financial control. Online pharmacies pay to enhance healthcare results and lower disparities in access to significant treatments.
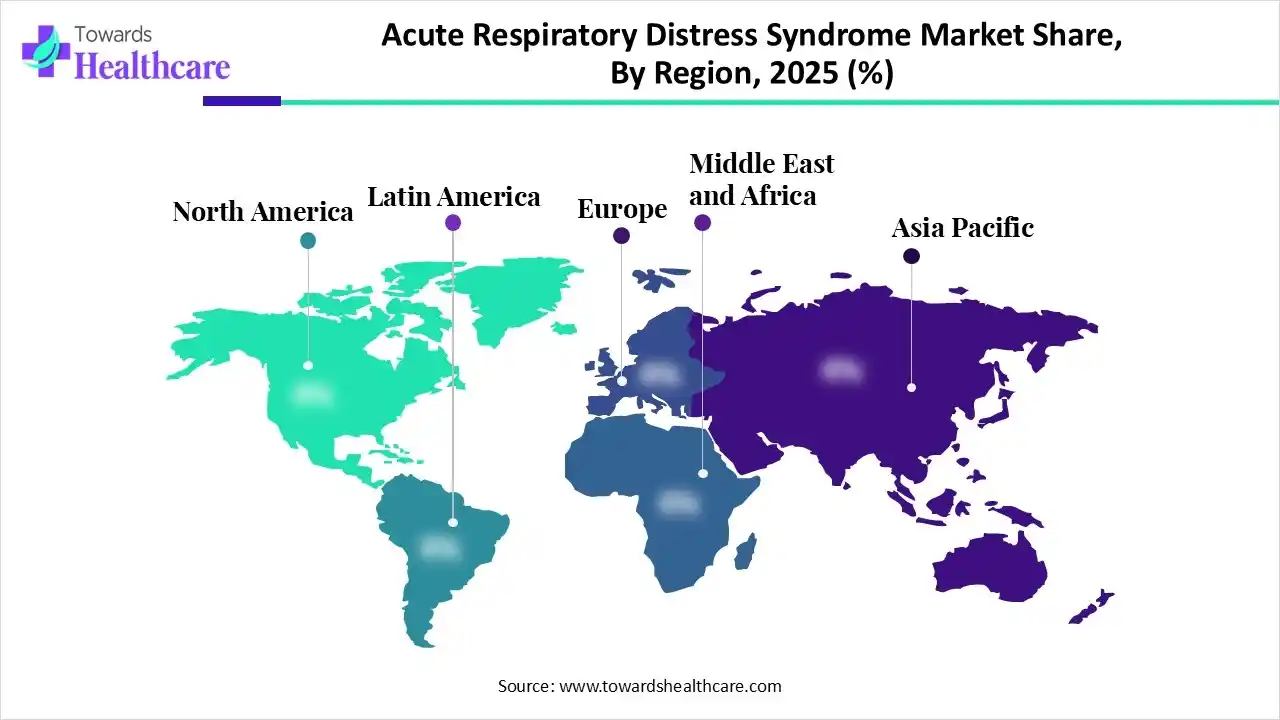
In 2025, North America led the acute respiratory distress syndrome market, as this region has the largest health system, which provides advanced treatment for acute respiratory distress syndrome. Rising prevalence and growing incidence of acute lung injury in North America. In this region, increasing clinical research tests novel treatments and therapies to regulate their safety, efficacy, and strength to improve health results, which drives the growth of the market.
U.S. Market Trends
In the U.S. increasing prevalence of chronic diseases, growing R&D spending in pharmaceuticals and biotechnology and rising demand for targeted medicine and recently developed treatments. The United States leads the world in progressive clinical research, driving innovations which figure out the future of medicalcare.
Asia Pacific is set to experience rapid growth in the acute respiratory distress syndrome market, as this region is a hub of a massive heterogeneous population whose respiratory health is influenced by varied social, economic, and environmental factors, which increases the incidence of acute respiratory distress syndrome, and thus increases the requirements advance treatment services. Around one-third of the global tobacco is produced and expended that growing the challenges of chronic lung conditions, which predispose patients to ARDS.
India Market Trends
In India, air pollution is a significant contributor to the disease load, which increases the cases of acute respiratory distress. Lower access to enhanced sanitation facilities is related to a massive risk of acute respiratory infections, driving the growth of the market.
Europe is experiencing substantial growth in the acute respiratory distress syndrome market, as European medicalcare systems reorient to enhanced public and population health. Public health is becoming a government priority, significantly focusing on early main care engagement to drive healthier lifestyles. Accepting developed digital technologies supports overcoming risks such as high cost and geography, confirming equitable access to services, which drives the growth of the market.
UK Market Trends
UK companies are revolution leaders in aspects of medical care infrastructure, from modelling, financing, and enterprise to construction and operation. The UK has the skills to support each aspect of infrastructure advancement. UK organizations offer a wide range of planning and modelling solutions required for successful, resource-effective infrastructure projects, contributes the growth of the market.
| Company | Headquarters | Latest Update |
| Athersys Inc. | United States | In December 2025, Athersys Inc. is enrolling a Pivotal Phase 2/3 clinical trial evaluating MultiStem cell therapy in COVID-19 induced and other pathogen-induced ARDS patients |
| mondoBIOTECH AG | Switzerland | MondoBIOTECH AG was previously involved in developing Aviptadil (RLF-100) for ARDS treatment. |
| HEALIOS K.K. | Japan | In December 2025, Healios KK announced its development policy for HLCM051, prioritizing its use as a treatment for ARDS. |
| GlaxoSmithKline plc. | United Kingdom | In May 2025, the U.S. Food and Drug Administration approved British drugmaker GSK's asthma drug to treat some patients with chronic lung diseases. |
| Pfizer Inc. | United States | In July 2025, Pfizer Inc. and BioNTech SE announced today that the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) has recommended marketing authorization for the companies’ LP.8.1-adapted monovalent COVID-19 vaccine for active immunization to prevent COVID-19 caused by SARS-CoV-2 in individuals 6 months of age and older. |
| Teva Pharmaceuticals USA Inc. | United States | In May 2025, Teva Pharmaceuticals, a U.S. affiliate of Teva Pharmaceutical Industries Ltd., and Alvotech announced that the U.S. Food and Drug Administration (FDA) had approved SELARSDI injection as interchangeable with the reference biologic Stelara. |
| Altor BioScience | United States | An AI tool was created to help identify the dangerous respiratory syndrome. |
Acute respiratory distress syndrome has a multifaceted pathophysiology that includes an intricate interplay of molecular and cellular processes, involving cytokine storms, programmed cell death, oxidative stress, and disruption of the alveolar-capillary barrier.
By Drug Class
By Route of Administration
By Distribution Channel
Regional Outlook
March 2026
February 2026
January 2026
January 2026
