January 2026

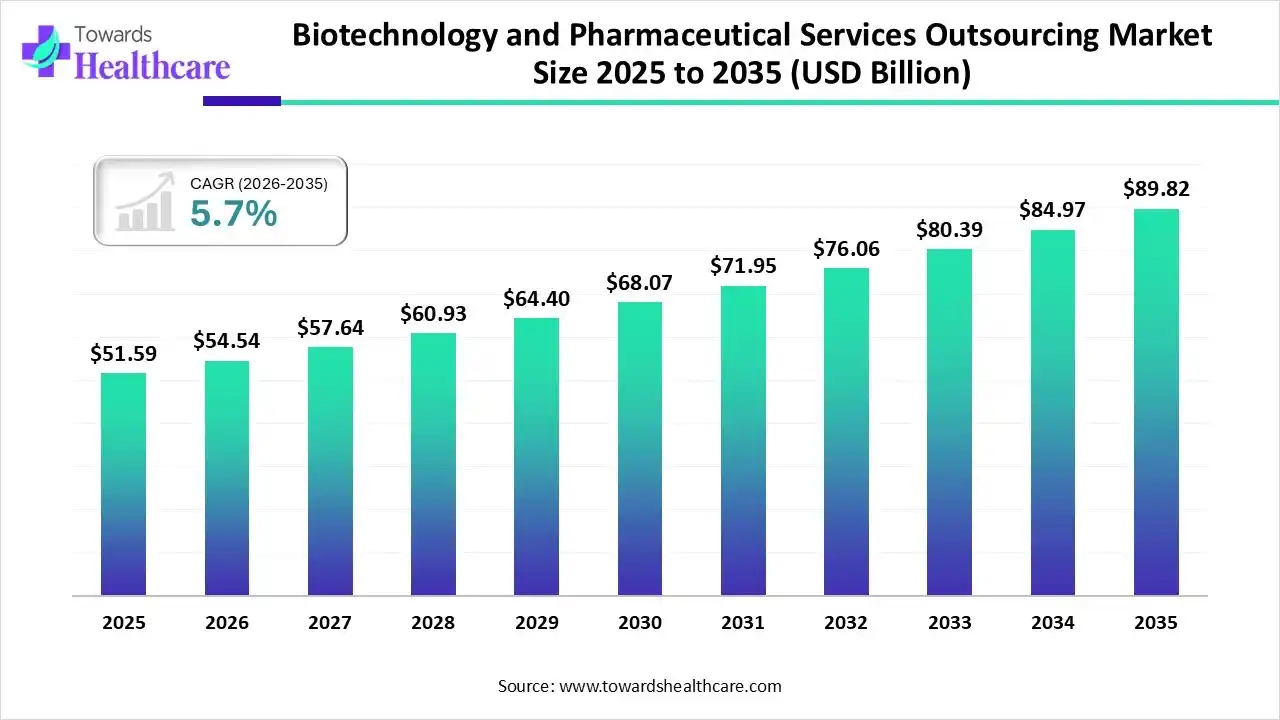
The global biotechnology and pharmaceutical services outsourcing market size was estimated at USD 51.59 billion in 2025 and is predicted to increase from USD 54.54 billion in 2026 to approximately USD 89.82 billion by 2035, expanding at a CAGR of 5.7% from 2026 to 2035.
The biotechnology and pharmaceutical services outsourcing market is growing due to the fact that outsourcing provides massive advantages to sponsor organizations, significantly lowers the requirement for capital investments, and provides growing access to dedicated technical expertise.
Outsourcing in biotech and pharma improves R&D processes, streamlines technology, and increases innovation via advanced solutions. As pharmaceutical and biotech organizations face mounting pressure to revolutionize while optimizing expenses, outsourcing providers incessantly improve their services to support R&D plans. These third-party organizations provide access to a large talent pool and are well-developed. Beyond the accelerating R&D, service outsourcing enables in-house teams to order high-value healthcare breakthroughs and evade project postponements.
The integration of AI-driven technology into biotechnology and pharmaceutical services outsourcing drives the growth of the biotechnology and pharmaceutical services outsourcing market, as the integration of AI-driven technology in outsourcing is most visible in how it basically enhances affordability, precision, and scalability. AI-based technology lowers reliance on manual labor, driving down operational expenses. Automation devices powered by AI handle massive volumes of information in real time, increasing turnaround. This strategic move towards AI integration in outsourcing is not easily related to delegating tasks; it’s about forging mergers that bring about innovation and drive sustainable biotechnology and pharmaceutical services growth.
| Key Elements | Scope |
| Market Size in 2026 | USD 54.54 Billion |
| Projected Market Size in 2035 | USD 89.82 Billion |
| CAGR (2026 - 2035) | 5.7% |
| Leading Region | North America |
| Market Segmentation | By Product, By End User, By Region |
| Top Key Players | Parexel International Corporation, The Quantic Group, Boston Scientific Corporation, IQVIA, Lachman Consultant Services, Inc., GMP Pharmaceuticals Pty Ltd. |
Which Service Led the Market in 2025?
In 2025, the consulting services segment held the dominant biotechnology and pharmaceutical services outsourcing market share, as biotech consulting plays a significant role for healthcare organizations, providing significant guidance on drug discovery, government compliance, and market access. This helps in streamlining the introduction of novel treatments. Biotech consulting includes significant guidance solutions tailored for biotechnology and drug organizations to navigate the intricate landscape of drug development.
Regulatory Affairs
Whereas the regulatory affairs segment is the fastest-growing in the market, as outsourcing provides a significant tool to rationalize operations, gain access to dedicated expertise, and hasten compliance processes. By outsourcing submission content advancement to government affairs consultants, biotech and pharmaceutical organizations can better mitigate risks, streamline procedures, and accelerate approval timelines.
Why did the Pharmaceutical Companies Segment Dominate the Market in 2025?
The pharmaceutical companies segment is dominant in the biotechnology and pharmaceutical services outsourcing market in 2025, as pharmaceutical companies have an advanced quality control system and testing measures well-organized in the system that avoids loss or wastage of resources, products, or efforts. It involves the growth and expansion of production processes, formulation advancement, and skill transfer. Pharmaceutical outsourcing affordable way to gather the resources and experts required to successfully carry out a clinical trial or programmes.
Biotechnology Companies
Whereas the biotechnology companies segment is the fastest growing in the market, as outsourcing biotechnology offers various benefits to sponsor companies, significantly reduced requirement for capital spending, and growing access to specialized technical expertise. Biotech organizations use a range of outsourcing strategies to optimize resources, streamline processes, and drive cost savings. It provides access to dedicated expertise and well-developed technologies that a company might not have in-house.
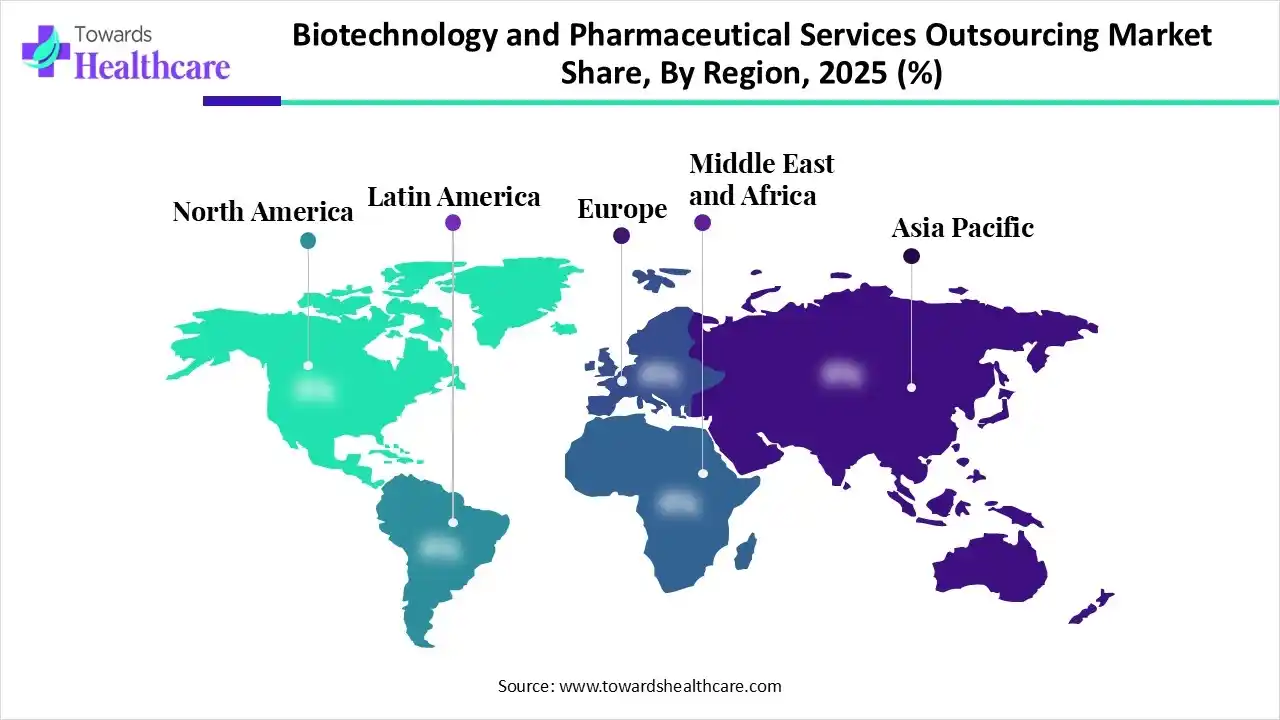
In 2025, North America led the biotechnology and pharmaceutical services outsourcing market, as increasing government funds have supported academic research, which in turn has increased private advancement, driving novel discoveries in healthcare, technology, and other sectors. Rising innovation economy and increasing access to it make a nation more competitive, grow economic advancement, address climate change, and improve health outcomes, which contribute to the growth of the market.
U.S. Market Trends
In the U.S. significant presence of leading research centres are keeping nation at the top in innovation, technology, and discoveries. The United States is significant in medical care spending in the developed biosphere. Novelty in healthcare is active in multiple R&D fields, involving the deployment of telemedicine, artificial intelligence and machine learning, 3D printing, wearable devices, antimicrobials, blockchain technology, and robotics, which drives the growth of the market.
Asia Pacific is set to experience rapid growth in the biotechnology and pharmaceutical services outsourcing market, due to the Governments in APAC having recognized the requirement to ramp up their efforts to increase the technological infrastructure of healthcare facilities. The government has placed healthcare as an important sector for investment and further development. The Asia-Pacific region, with its massive population, is a top choice for clinical trials due to lower costs and a large patient base, this drive the growth of the market.
India Market Trends
India offers 60-80% cost savings in outsourcing as compared to hiring in the U.S., particularly for roles such as data entry, virtual assistants, and consumer support. India has the highest number of USFDA-approved manufacturing plants worldwide, with 499 amenities. Indian CDMOs and CROs are growing, focusing on high-value and innovative products.
Europe is experiencing substantial growth in the biotechnology and pharmaceutical services outsourcing market, as European pharma is developing worldwide medical care through innovation, advanced treatments, and sustainable services for a healthier world. Medical technology fields are involved in advancing the EU agenda by growing innovation, enhancing public health, and driving economic growth.
UK Market Trends
Increasing medical care innovation in the UK covers a various ecosystem spanning biopharma, healthtech, and medtech. The United Kingdom is Europe's leading biotech hub in breakthrough life sciences start-ups. The UK’s biotech sector stands as the most vibrant in the world, heavily supported by an advanced research base and a solid network of funding agencies.
| Country | Clinical Trials |
| United State | 197090 |
| China | 162704 |
| India | 94141 |
| Japan | 67462 |
| Germany | 59320 |
| United Kingdom | 52227 |
| France | 50768 |
| Company | Headquarters | Latest Update |
| Parexel International Corporation | United States | This organization provides services for managing the biopharmaceutical product lifecycle and the development and commercialization of novel healthcare therapies. |
| The Quantic Group | New Jersey | The Quantic Group is a leading worldwide consultancy specializing in the pharmaceutical, biotechnology, and medical device fields. |
| Boston Scientific Corporation | United States | The Quantic Group is a pharmaceutical, biotechnology, vaccine, and medical device specialty consulting firm that supports both the industry and governments |
| IQVIA | Germany | In June 2025, IQVIA, a leading global provider of clinical research solutions, commercial insights, and medical care intelligence to the life sciences and healthcare industries, unveils AI agents at GTC Paris |
| Lachman Consultant Services, Inc. | United State | Lachman Consultant Services, Inc. offers specialized biotech and pharma outsourcing via expert government, compliance, and scientific guidance |
| GMP Pharmaceuticals Pty Ltd. | Australia | GMP Pharmaceuticals Pty Ltd is a leading Australasian contract manufacturer of complementary medicines, natural health products, and infant formulas. |
By Product
By End User
By Region
January 2026
January 2026
January 2026
January 2026