February 2026

The cell therapy solvent suspension tubing market is experiencing significant expansion, with projections indicating a revenue increase reaching several hundred million dollars by the end of the forecast period, spanning 2026 to 2035. This growth is driven by emerging trends and strong demand across key sectors. The large-scale clinical and manufacturing applications of cell suspensions and tubing maintain safety and sterility in processing.
The cell therapy solvent suspension tubing market is accelerated by the rising trends and innovations in cell therapy manufacturing, which include automation, closed systems, scalable expansion, material compatibility, and single-use technologies. The industries are prioritizing heavy investments in automated and closed-loop systems to reduce contamination risks and improve efficiency. They also focus on the integration of pre-sterilized, highly reliable, and single-use tubing sets into bioreactors and processing equipment. The major players in cell therapy technologies are Terumo Blood and Cell Technologies, Borosil Scientific, Abdos Life Sciences, and Avantor/VWR, focusing on the launch of specialized products and materials for manufacturing and processing.
AI contributes to automation, process optimization, and real-time quality control across the cell therapy manufacturing sector. AI revolutionizes the cell therapy solvent suspension tubing market by assisting in reducing costs, improving efficiency, and increasing the safety of therapeutic products. AI-driven systems play a major role in process automation and monitoring, automated image analysis, anomaly and contamination detection, and process design and simulation.
How does the Thermoplastic Elastomer (TPE) Segment Dominate the Cell Therapy Solvent Suspension Tubing Market in 2025?
The thermoplastic elastomer (TPE) segment dominated the market in 2025, owing to its durability, stability, and flexibility, and the wide use in the biotechnology and biopharmaceutical sectors. The TPE tubing serves as a reliable solution to transport delicate substances such as vaccines, biologics, and cell cultures. The TPE allows recycling options, which reduces waste related to single-use systems, and meets sustainability goals.
Silicone
The silicone segment is expected to grow at the fastest CAGR in the cell therapy solvent suspension tubing market during the forecast period due to its biocompatibility, non-toxicity, chemical inertness, and purity. It is flexible, durable, and maintains the purity and viability of sensitive cell suspensions. It is essential for quality control in laboratory and pharmaceutical settings.
What made Biopharma & Biotechnology Companies the Dominant Segment in the Cell Therapy Solvent Suspension Tubing Market in 2025?
The biopharma & biotechnology companies segment dominated the market in 2025, owing to the major role and applications of cell suspension tubing in aseptic fluid transfer and contamination control. These industries focus on process monitoring and automation support through the seamless integration of tubing systems. They prioritize cell viability, product integrity, cryopreservation, storage, scalability, and flexibility.
CMOs/CDMOs
The CMOs/CDMOs segment is estimated to grow at the fastest rate in the cell therapy solvent suspension tubing market during the predicted timeframe due to the new approaches towards closed and single-use manufacturing systems. The CDMOs and CMOs focus on improving efficiency, ensuring sterility, and meeting stringent regulatory requirements for cell and gene therapies. They also make efforts to simplify regulatory inspections by improving traceability and reducing bioburden.
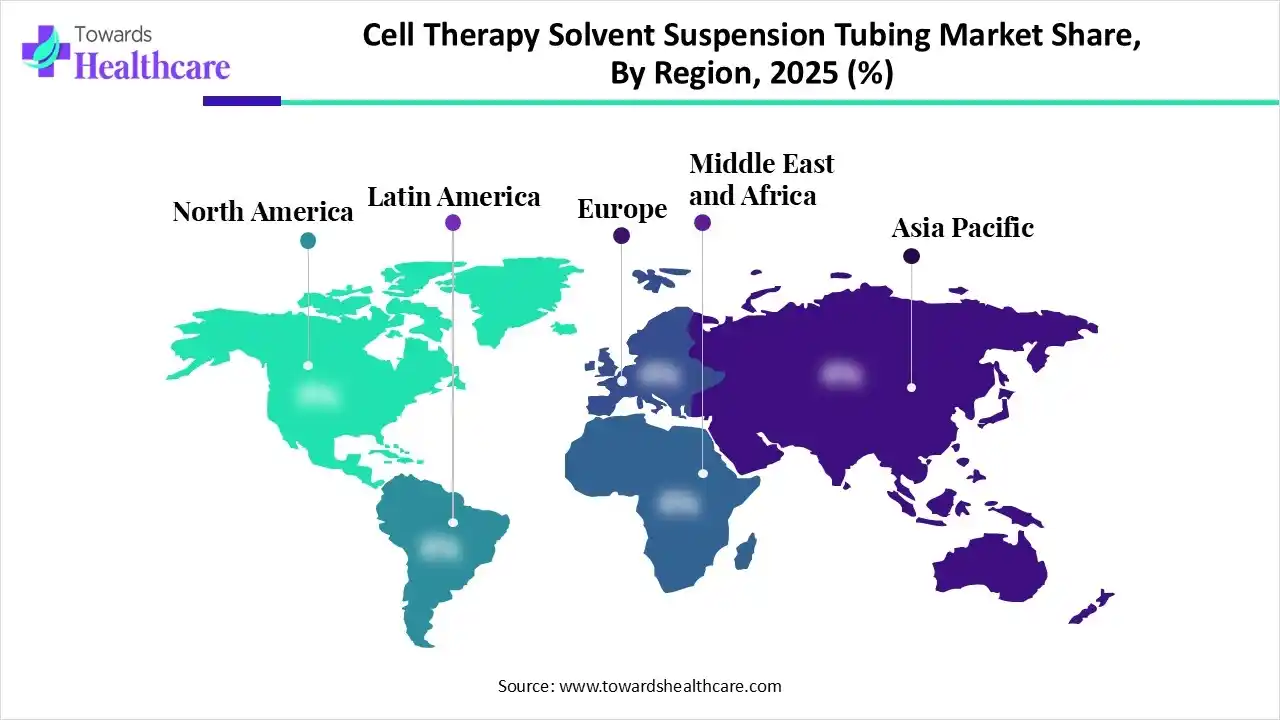
North America dominated the market in 2025, owing to the rising demand for advanced therapies, focus on quality and contamination control, and the increased number of clinical trials. The North American cell therapy solvent suspension tubing market is experiencing massive growth due to certain initiatives by the government. In September 2025, the Advanced Research Projects Agency for Health (ARPA-H) launched a new program to boost innovative biomanufacturing for cell and gene therapies.
The U.S. Department of Health and Human Services (HHS) offered funding through its Genetic Medicines and Individualized Manufacturing for Everyone program. This program aims to position the U.S. in advanced manufacturing methods for high-quality genetic medicines.
Asia Pacific is expected to grow at the fastest CAGR in the market during the forecast period due to advancements in regenerative medicine and technology, and improving regulatory frameworks. According to Thermo Fisher Scientific Inc., cell and gene therapies are gaining momentum in the Asia Pacific and driving the cell therapy solvent suspension tubing market due to the rising patient demand and innovation. This region is committed to resolving challenges arising in this field to enable the success of cell and gene therapy products.
China leads the position in advanced therapies across this region due to the rising incidence of solid tumors. The increased government and regulatory support for cell and gene therapy trials drives the country. There are significant opportunities for drug developers and patients driven by the country’s expansion.
Europe is expected to grow at a notable rate in the market in 2025, led by increasing R&D investments and demand for personalized medicine. In May 2024, the European Commission approved a project on cell and gene therapy. The European Commission and the European Medicines Agency (EMA) are involved in regulatory advancements for advanced therapy medicinal products. Europe supports and regulates the manufacturing and delivery of advanced cell therapies.


| Sr. No. | Name of the Company | Headquarter | Latest Update |
| 1 | Thermo Fisher Scientific | Waltham, Massachusetts, USA | In September 2025, Thermo Fisher Scientific completed the acquisition of Solventum’s purification and filtration business. |
| 2 | Sartorius AG | Göttingen, Germany | In October 2025, Sartorius AG reported its profitable sales revenue growth of up to 7.5% in the first nine months. |
| 3 | Merck KGaA | Darmstadt, Germany | In July 2024, Merck signed an MoU with Gene Therapy Research Institution Co., Ltd. to produce viral vector-based gene therapy for Parkinson’s Disease. |
| 4 | Avantor | Radnor, Pennsylvania, USA | In October 2025, Avantor and BlueWhale Bio Partner planned to boost CAR-T manufacturing with an innovative cell activation and expansion technology. |
| 5 | RAUMEDIC | Helmbrechts, Germany | In August 2025, RAUMEDIC launched an innovative silicone tubing for increased application pressure in bio-pharma tubing. |
| 6 | Terumo Corporation | Shibuya-ku, Tokyo, Japan | In November 2025, Terumo Blood and Cell Technologies Quantum Flex Bioreactor introduced the first-ever automated 3-in-1 TCR-T manufacturing to the University of Chicago School of Medicine. |
| 7 | Lonza Group AG | Basel, Switzerland | In October 2025, Lonza expanded its product portfolio of TheraPEAK with the inclusion of AmpliCell cytokine range and TheraPEAK 293-GT medium. |
| 8 | Parker Hannifin | Cleveland, Ohio, USA | In November 2025, Parker Hannifin launched a new gap filler gel from Parker Chomerics with very high performance. |
| 9 | Danaher Corporation | Washington, D.C., U.S. | Researchers at the University of Pennsylvania and Danaher planned to improve the use of lipid nanoparticles to enable new CAR T therapies for blood cancers |
| 10 | Saint-Gobain Performance Plastics | La Défense, France | In July 2025, Qosina expanded Saint-Gobain's product offering through a new distribution partnership to manufacture high-performance tubing and polymer solutions. |
By Material Type
By End-User
By Region
February 2026
January 2026
January 2026
January 2026