January 2026

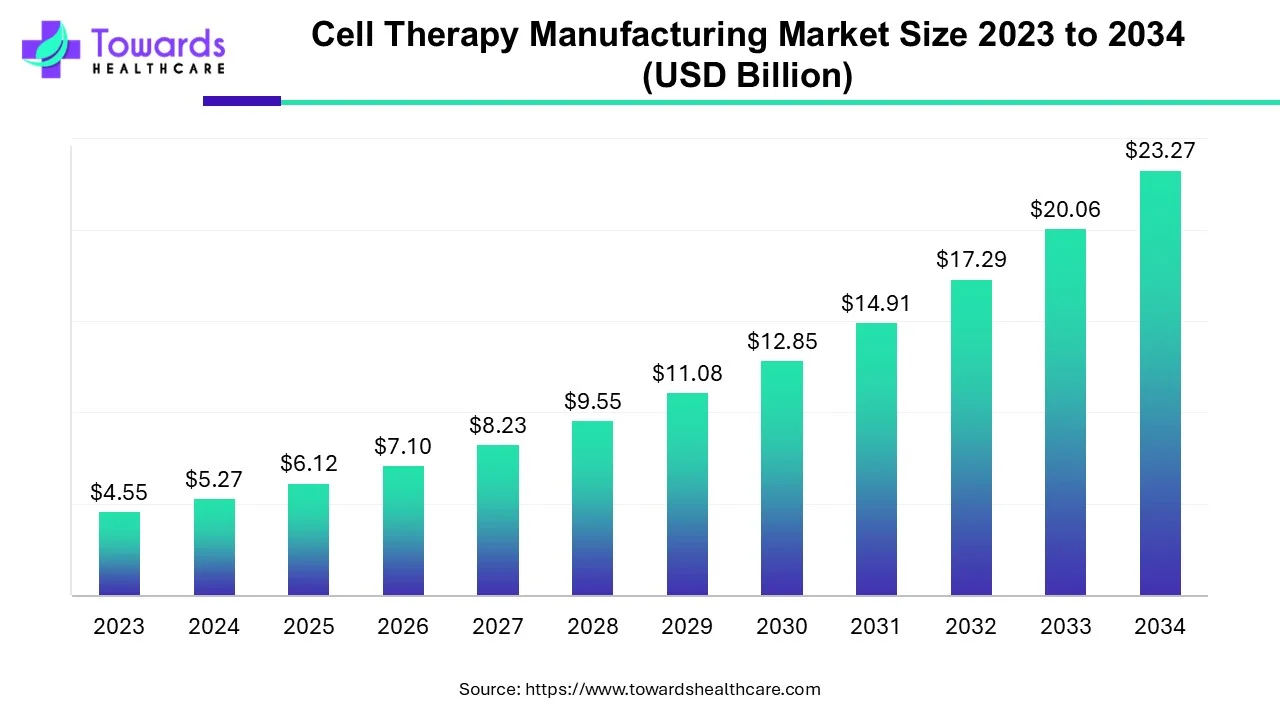
The global cell therapy manufacturing market is estimated to grow from USD 6.12 billion in 2025 at 16% CAGR (2025-2034) to reach an estimated USD 23.27 billion by 2034.
Nearly all cancer diagnoses, accounting for 90% of cases, require cell therapies to effectively treat solid tumors.
Cell therapy manufacturing refers to the process of producing therapeutic cells that are used for the treatment of various diseases and medical conditions. It involves the isolation, expansion, modification, and quality control of cells in order to generate a sufficient number of functional cells for therapeutic use. Cell therapies have gained significant attention in recent years due to their potential for treating a wide range of diseases, including cancer, cardiovascular disorders, neurodegenerative diseases, and autoimmune disorders. The manufacturing process for cell therapies is complex and requires specialized infrastructure, equipment, and expertise. It typically involves the use of advanced techniques such as cell culture, genetic engineering, and quality control testing. The cells used in therapy can be derived from different sources, including autologous (patient's own cells) or allogeneic (donor-derived) cells.
The driving factors for the cell therapy manufacturing market include the increasing demand for personalized medicine, advancements in cell therapy research and development, and the growing prevalence of chronic diseases. Cell therapies offer the potential for targeted and individualized treatments, which can lead to improved patient outcomes. Additionally, the rising investments in regenerative medicine and the favorable regulatory environment for cell therapies further drive market growth.
| Metric | Details |
| Market Size in 2024 | USD 5.27 Billion |
| Projected Market Size in 2034 | USD 23.27 Billion |
| CAGR (2025 - 2034) | 16% |
| Leading Region | North America |
| Market Segmentation | By Therapy, By Technology, By Source, By Application and By Region |
| Top Key Players | Merck KGaA, Thermo Fisher Scientific, Catalent, Inc, Bio-Techne, Cytiva, Lonza, The Discovery Labs |
Artificial intelligence (AI) revolutionizes the market by introducing automation in the manufacturing process. AI can aid in the large-scale manufacturing of cell therapy products, improving efficiency, accuracy, and reproducibility. AI also helps to reduce the overall cost of production and minimize human errors. Hence, AI and machine learning (ML) can optimize the cell therapy production process. AI-enabled robots are the future of cell therapy manufacturing, facilitating homogenous production, and reducing variability. AI can also aid in streamlining the research process for developing novel cell therapy products. It can predict the function and potency of cell therapy products, improving their characterization and optimization.
The cell therapy manufacturing market is witnessing a significant increase in collaborations and expansion activities by market players, which is driving its growth. Collaborations between academic institutions, research organizations, and industry players are becoming increasingly common in the field of cell therapy manufacturing. These partnerships aim to combine resources, expertise, and technologies to accelerate the development and commercialization of cell therapies.
Through collaborations, companies can access a broader range of knowledge and capabilities, enabling them to overcome technical challenges and optimize their manufacturing processes. They can also leverage the expertise of academic and research institutions to enhance their understanding of cell biology, process optimization, and quality control. By pooling resources and sharing risks, collaborations help to expedite the translation of cell therapies from the laboratory to the clinic. For instance.
In addition to collaborations, market players are expanding their manufacturing capabilities through facility expansions and acquisitions. The growing demand for cell therapies and the need for increased production capacity is driving companies to invest in larger and more advanced manufacturing facilities. These expansions not only enable companies to meet the rising demand for cell therapies but also provide them with the infrastructure and resources to support further research and development.
For instance,
Expanding into new geographies is another strategy adopted by market players to capitalize on emerging markets and gain a competitive edge. By establishing manufacturing facilities or partnerships in different regions, companies can cater to local patient populations, leverage regional expertise, and comply with specific regulatory requirements. This expansion allows companies to access new markets, increase their market share, and diversify their revenue streams.
The rising collaborations and expansions in the cell therapy manufacturing market are driven by several factors. Firstly, the increasing demand for cell therapies and the growing pipeline of cell therapy candidates are creating opportunities for market players to scale up their manufacturing operations. The potential of cell therapies to revolutionize healthcare and provide innovative treatment options is attracting significant investments and industry interest.
Clinical trials play a crucial role in driving market growth in the field of cell therapy manufacturing. Clinical trials are conducted to assess the safety and efficacy of cell therapies in humans. These trials provide valuable data on the therapeutic potential, dosage, administration, and potential side effects of cell therapies. Positive results from clinical trials contribute to market growth by building confidence among healthcare professionals, regulatory authorities, and patients. Clinical trials are a prerequisite for obtaining regulatory approvals for cell therapies. Regulatory agencies, such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), require robust clinical trial data to evaluate the safety and efficacy of cell therapies before granting market authorization. Successful completion of clinical trials and obtaining regulatory approvals pave the way for commercialization and market entry. The rising number of clinical trials significantly augments the growth of this market.
In addition, clinical trials enable researchers and developers to explore new indications and expand the application of cell therapies. Through well-designed clinical trials, the efficacy of cell therapies can be evaluated in various disease areas, leading to the expansion of treatment options and addressing unmet medical needs. The identification of new indications and successful clinical trial outcomes contribute to market growth. Positive outcomes from clinical trials provide a competitive advantage to cell therapy manufacturers. Demonstrating the safety and efficacy of their therapies through well-designed clinical trials helps companies differentiate themselves from competitors and gain market share. Clinical trial data strengthens the credibility of cell therapies and enhances their market appeal among healthcare providers, payers, and patients. Increasing product launches and product approvals extensively bolster the growth of the cell therapy manufacturing market.
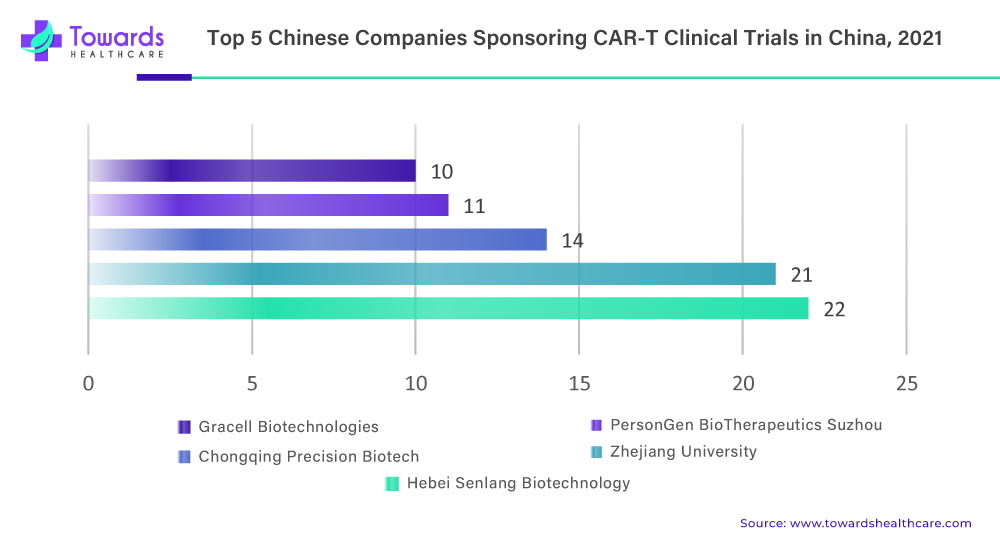
In Beijing, CAR-T research involves various entities such as universities, clinical researchers, and small start-up companies. However, many of these entities lack the infrastructure to build their own manufacturing capacity. To address this issue, the Beijing government has set up a centralized production line in the metropolitan science and technology park. This facility offers manufacturing space that can be rented by researchers and start-ups, aiming to lower their research and development costs. This initiative provides a solution for entities involved in CAR-T research to access manufacturing capabilities and promote the advancement of their therapies.
Clinical trials are essential drivers of the cell therapy manufacturing industry. They provide evidence of safety and efficacy, support regulatory approvals, facilitate treatment optimization and innovation, differentiate products in the market, attract investors, and enable the exploration of new indications. Continued investment in well-designed clinical trials is crucial for the growth and advancement of the cell therapy manufacturing sector.
Developing cell therapies requires extensive research and development efforts, including preclinical studies and early-phase clinical trials. These activities involve significant investment in laboratory equipment, personnel, materials, and specialized facilities. Establishing the necessary manufacturing infrastructure for cell therapies can be costly. This includes cleanroom facilities, specialized equipment, and quality control systems to ensure the safety and efficacy of the manufactured products. The costs of maintaining and operating these facilities add to the overall development expenses.
Conducting clinical trials is a resource-intensive process. Patient recruitment, treatment administration, monitoring, data collection, analysis, and reporting all contribute to the high costs. In addition, complying with regulatory requirements and ensuring patient safety through rigorous monitoring and adverse event reporting further adds to the expenses. Scaling up cell therapy production from small-scale clinical trials to large-scale commercial manufacturing involves substantial costs. The costs associated with process optimization, facility expansion, and quality control measures increase as the production volume increases.
Meeting regulatory standards and ensuring product quality and safety throughout the manufacturing process require robust quality control measures. These measures can be expensive to implement and maintain, including testing, validation, documentation, and compliance with good manufacturing practices (GMP) regulations. Protecting intellectual property rights through patents and licensing agreements is crucial in the cell therapy field. Securing and maintaining intellectual property rights involves legal fees and ongoing costs, which can be significant.
The high development costs pose challenges for both industry players and academic institutions, especially for small and emerging companies with limited financial resources. These costs may deter some companies from entering the market or hinder the progress of research and development initiatives.
To address this restraint, it is important to explore strategies to optimize the development process, improve efficiency, and reduce costs. Collaboration between industry, academia, and regulatory bodies can help in streamlining regulatory requirements and fostering an environment conducive to cost-effective development. Additionally, investments in research and development funding, government incentives, and public-private partnerships can support the advancement of cell therapy manufacturing and mitigate the financial burden on stakeholders.
Advancements in technology and manufacturing processes have played a significant role in unlocking opportunities in the cell therapy market. These advancements have revolutionized the field of cell therapy, enabling the development and manufacturing of innovative and complex therapies. Technological advancements have led to the development of automated and closed-system manufacturing platforms, allowing for the production of cell therapies at a larger scale. This scalability improves efficiency, reduces costs, and increases the availability of cell therapies for a broader patient population.
Advanced bioreactors, monitoring systems, and analytics tools have enabled better process control and optimization in cell therapy manufacturing. This optimization improves product quality, consistency, and reproducibility, reducing the risk of batch failures and increasing the overall success rate of cell therapies. innovations in gene editing technologies, such as CRISPR-Cas9, have opened up new possibilities for modifying and engineering cells used in therapies. This technology allows for precise and targeted modifications of cell genomes, enhancing therapeutic efficacy and expanding the scope of cell therapies.
Cryopreservation technologies have improved the long-term storage and preservation of cells, enabling their use in off-the-shelf therapies. Cryopreserved cell products offer convenience and flexibility in clinical settings and facilitate wider adoption of cell therapies. Advances in technology have facilitated the development of combination therapies that combine different cell types, gene therapies, or small molecules. These synergistic approaches have the potential to enhance therapeutic outcomes and address complex diseases more effectively. Technological advancements, such as high-throughput screening and next-generation sequencing, have enabled the characterization of patient-specific cells and identification of biomarkers. This personalized approach allows for the development of tailored cell therapies and more precise treatment strategies.
Thus, advancements in technology and manufacturing processes have revolutionized the cell therapy market, offering numerous opportunities for innovation and growth. By leveraging these advancements, companies can enhance the scalability, cost-efficiency, and efficacy of cell therapies, driving the field forward and improving patient outcomes.
By therapy, the autologous cell therapy segment held a dominant presence in the cell therapy manufacturing market in 2024. Autologous cell therapy refers to cell therapy in which cells are collected from the patient, processed ex vivo, and then transferred back to the same patient. Incorporating autologous cells into the patients eliminates the risk of immune response. This facilitates a higher chance of success and fewer side effects, promoting long-term healing. The lack of availability of immediate donors promotes the use of autologous cell therapy.
By therapy, the allogeneic cell therapy segment is expected to grow at the fastest rate in the market during the forecast period. Allogeneic cell therapy refers to a technique in which patients receive stem cells from healthy donors. The major advantage of using allogeneic cell therapy is the elimination of co-morbidities associated with disease states. The increasing number of stem cell banks and the latest innovations in storage equipment potentiate the segment’s growth.
By technology, the somatic cell technology segment led the global cell therapy manufacturing market in 2024. Somatic cell nuclear transfer (SCNT) is a lab technique for creating a viable embryo from a body cell and an egg cell. This technology is used to obtain pluripotent cells from a cloned embryo. SCNT is also used to generate tissues or organs for transplant into a specific patient. The growing research and development activities and advancements in technology propel the segment’s growth.
By technology, the 3D technology segment is anticipated to grow with the highest CAGR in the market during the studied years. 3D printing is an emerging technology that is used to design and manufacture biological products, including cell therapy. It aids in faster, more accurate, and cost-effective cell therapy manufacturing. Integrating 3D printing with cell therapy provides personalized, cell-laden constructs that mimic the complex architecture and function of native tissues.
By source, the induced pluripotent stem cells (iPSCs) segment registered its dominance over the global market in 2024. iPSCs are cells that can be generated directly from a somatic cell. They are widely used to model human development and diseases, perform drug screening, and develop cell therapies. The demand for iPSCs is increasing as they can propagate indefinitely and give rise to any cell type of the body.
By source, the bone marrow segment is estimated to show lucrative growth in the market over the forecast period. Stem cells that are extracted from bone marrow have the capacity to transform into red blood cells, white blood cells, or platelets. The rising incidences of hematological disorders promote the segment’s growth.
By application, the oncology segment held the largest share of the cell therapy manufacturing market in 2024. The segmental growth is attributed to the increasing incidences and prevalence of cancer, increasing investments in cancer research, and favorable regulatory frameworks. The U.S. Food and Drug Administration (FDA) approved 7 cell and gene therapy products.
By application, the neurological segment is projected to expand rapidly in the market in the coming years. The rising incidences of neurological disorders and their complexity necessitate the development of cell therapy. The increasing geriatric population and new product launches potentiate the segment’s growth. The U.S. FDA approved a novel cell therapy product for Alzheimer’s disease in 2023.
North America, particularly the United States, holds a significant share in the cell therapy manufacturing market. The region benefits from advanced healthcare infrastructure, a well-established regulatory framework, and a strong presence of key market players. The United States, in particular, has been at the forefront of cell therapy research and development, attracting substantial investments and fostering collaborations between industry, academia, and research institutions.
The U.S. market is growing rapidly due to strong regulatory support, rising chronic and genetic disease prevalence, and technological advancements. Expedited FDA pathway and incentives like RMAT designation have accelerated development. Innovations in automated manufacturing and strategic partnerships, such as those involving Thermo Fisher, are improving scalability and reducing costs. These factors, combined with increasing demand for personalized treatments, are driving the market growth.
Canada’s market is expanding due to strong government support, including significant funding for commercialization centers like CCRM. Advancements in cell biology and scalable manufacturing technologies are driving innovation. Additionally, collaboration between research institutions and biotech companies, along with new manufacturing infrastructure, is enhancing the country’s capacity for cell therapy development and production.
In addition, Europe is another prominent region in the cell therapy manufacturing market. Countries such as Germany, the United Kingdom, and France have a robust healthcare system and a supportive regulatory environment for cell therapies. The European Medicines Agency (EMA) provides guidelines and regulations for the development and commercialization of cell therapies, ensuring safety and efficacy. The region also witnesses collaborations and partnerships among academic institutions, hospitals, and industry players to advance cell therapy manufacturing.
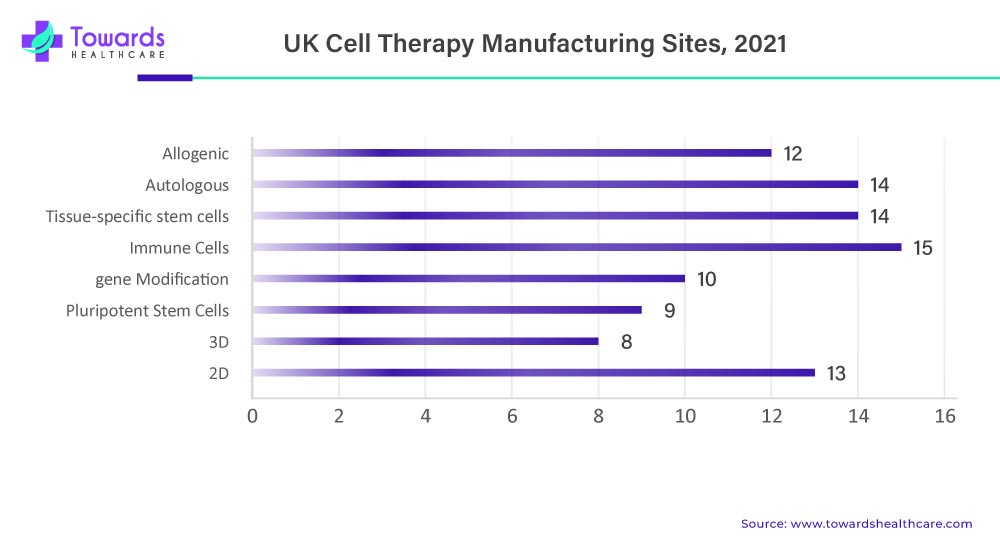
The UK market is growing due to expanded GMP infrastructure, including facilities like Autolus’ The Nucleus. Technological advancements like gene editing and bioreactor systems have improved efficiency and scalability. Strategic mergers and acquisitions have strengthened expertise and production capacity. Additionally, the UK’s alignment with global regulatory standards has encouraged international collaboration, making it an attractive hub for innovation and manufacturing in the cell therapy space.
Germany’s market is expanding due to government initiatives like the Biotech Strategy 2025 and significant R&D funding. Advanced technologies such as CRISPR and closed-system bioreactors enhance efficiency and scalability. A robust regulatory framework ensures safety and encourages investment. Strategic collaboration between academic institutions and industry leaders, like Evotec and Novo Nordisk, further drives innovation and production capacity in the cell therapy sector.
Cell Therapy Manufacturing Facilities in the U.K.
Furthermore, the Asia-Pacific region is experiencing significant growth in the cell therapy manufacturing market. Countries like China, Japan, and South Korea are actively investing in research and development and building advanced manufacturing facilities. The region benefits from a large patient population, increasing healthcare expenditure, and supportive government initiatives to promote the development and adoption of cell therapies. Asia-Pacific is also emerging as a hub for contract manufacturing organizations (CMOs) offering cell therapy manufacturing services.
China’s market is growing due to government support, like the “Made in China 2025” plan. The country’s streamlined regulatory processes, including alignment with international standards, foster quicker development. China leads in clinical trials, especially CAR-T therapies. Moreover, increasing foreign investment and collaboration, like AstrZeneca’s acquisition of Gracell, further solidify its role in the global biotech sector.
India’s market is expanding due to advancements in biotechnology and regenerative medicines, which drive the development of innovative therapies. The country’s diverse population provides unique opportunities for clinical trials, particularly for diseases prevalent in the region. Additionally, India’s well-established pharmaceutical infrastructure enables cost-effective Production of cell therapies, making it an attractive player in the global biotechnology sector.
| Name | Trade Name | Manufacturer | Cell Source | Approval Year |
| Tisagenlecleucel | Kymriah | Novartis | Autologous | 2017 (USFDA), 2018 (EMA), 2018 (Health Canada), 2019 (JMHW), Australia, Israel, Switzerland |
| Axicabtagene ciloleucel | Yescarta | Kite | Autologous | 2017 (USFDA), 2018 (EMA), 2019 (Health Canada) |
| Brexucabtagene autoleucel | Tecartus | Kite | Autologous | 2020 (USFDA) |
Dr. Dan Strange, CTO at Cellular Origins, commented at the ELRIG Drug Discovery 2024 conference that limited cell therapy manufacturing capacity is a major concern, resulting in tens of thousands of patients not receiving therapies that could be curative. He envisioned that incorporating automation technologies into manufacturing would solve such issues.

By Therapy
By Technology
By Source
By Application
By Region
January 2026
January 2026
January 2026
January 2026