January 2026

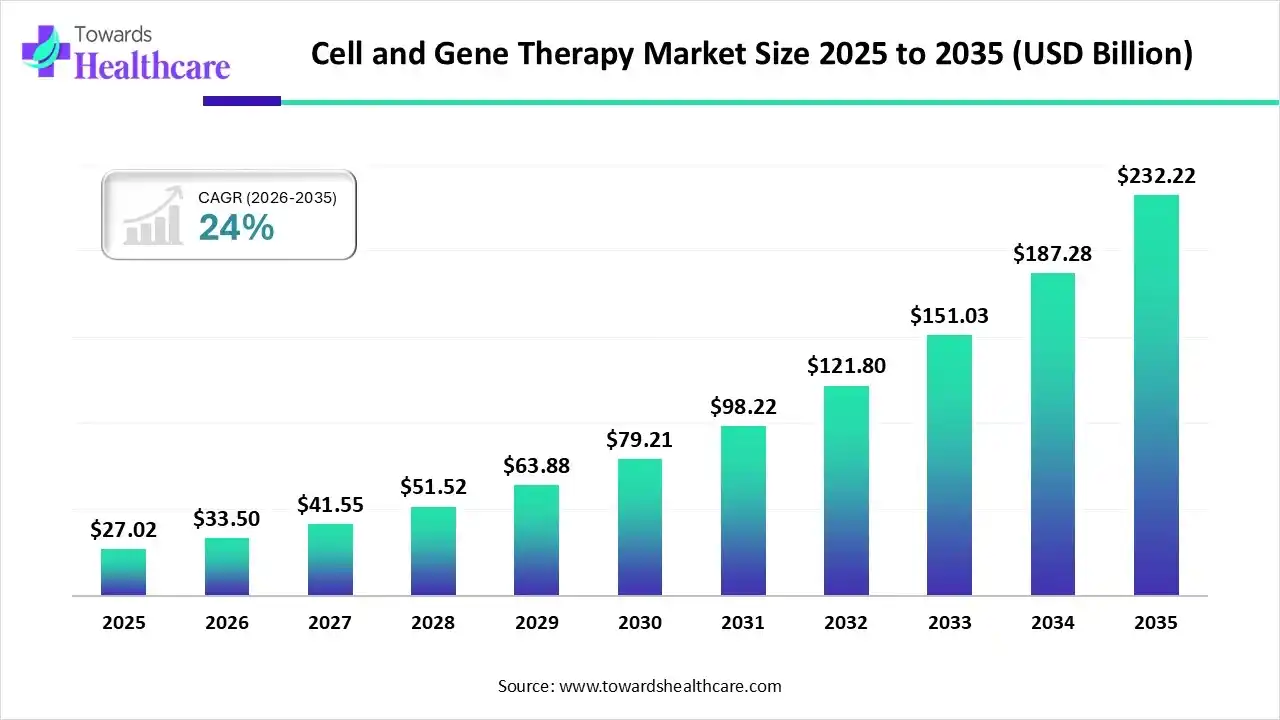
The cell and gene therapy market size touched US$ 27.02 billion in 2025, with expectations of climbing to US$ 33.5 billion in 2026 and hitting US$ 232.22 billion by 2035, driven by a CAGR of 24% over the forecast period.
| Key Elements | Scope |
| Market Size in 2026 | USD 33.5 Billion |
| Projected Market Size in 2035 | USD 232.22 Billion |
| CAGR (2026 - 2035) | 24% |
| Leading Region | North America |
| Market Segmentation | By Therapy Type, By Therapeutic Class, By Delivery Method, By End-Users, By Region |
| Top Key Players | Alnylam Pharmaceuticals Inc., Amgen Inc., Biogen Inc., CORESTEM Inc., Dendreon Pharmaceuticals LLC., Helixmith Co. Ltd., JCR Pharmaceuticals Co. Ltd., Kolon TissueGene Inc., Novartis AG, and Pfizer Inc. |
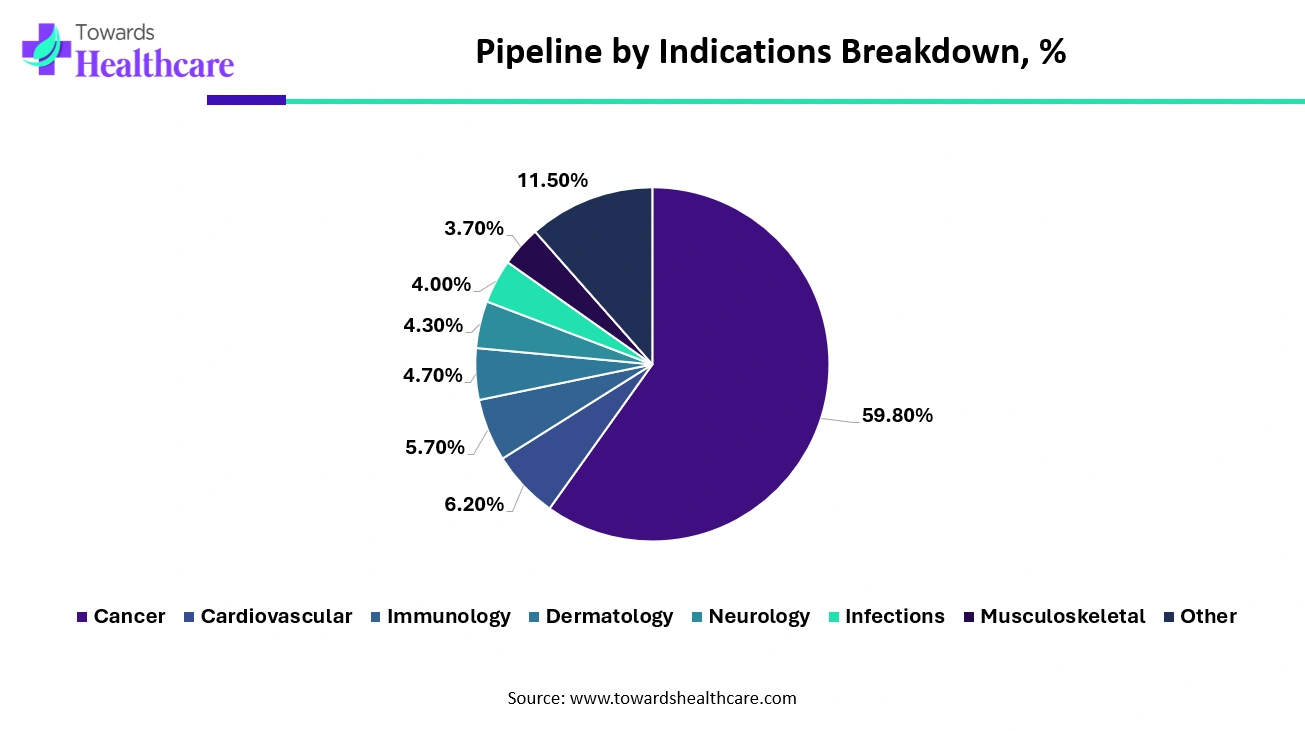
Oncology Accounts for the Most Active Therapeutic Segment Through All the Stages of Development.
The cell and gene therapy market is a rapidly growing sector within the healthcare industry that involves the use of cellular and genetic materials to treat various diseases and medical conditions. Cell therapy involves the transplantation or administration of live cells into a patient's body to restore or improve cellular function, while gene therapy involves the introduction or alteration of genetic material to treat genetic disorders or modify the function of cells.
Cell and gene therapies have the potential to revolutionize the treatment of a wide range of diseases. They can be used for conditions such as cancer, genetic disorders, cardiovascular diseases, neurodegenerative diseases, and autoimmune disorders, among others. This broad applicability enhances the market's growth potential.
Oncology continued to be the most active therapeutic area of research across all stages of development, including preclinical through preregistration. In the field of gene and cell therapy, there were more than 1,300 candidates reported in development specifically for oncology in 2021. This highlights the significant focus and investment in developing innovative therapies to address cancer and improve patient outcomes.
Explanations:
Rising Prevalence of Chronic Diseases
The increasing number of people suffering from chronic illnesses such as cancer, genetic disorders, and autoimmune diseases is significantly driving the growth of the cell and gene therapy market. These advanced therapies offer targeted and potentially curative solutions by addressing the underlying genetic or cellular causes of disease, unlike traditional treatments that often only manage symptoms. With a rising global patient population and growing demand for more effective, long-term treatments, cell and gene therapies are becoming essential tools in modern medicine, offering hope where conventional methods have limited success.
High-Cost Associated Therapies
The high cost of cell and gene therapies is a major factor limiting market growth. These treatments often involve complex development, personalized manufacturing, and rigorous clinical testing, all of which drive up costs. As a result, many patients and healthcare systems face affordability challenges. Limited insurance coverage and high out-of-pocket expenses further restrict access, especially in low- and middle-income regions. This financial barrier slows widespread adoption, despite the therapies' medical potential.
Expansion of Cell and Gene Therapy Manufacturing
The expansion of manufacturing capacities presents a key opportunity for the cell and gene therapy market because it helps meet growing demand, lowers production costs, and improves accessibility. With more facilities and advanced technologies, companies can produce therapies at a larger scale, more efficiently, and with consistent quality. This also supports faster delivery of treatments, better patient access, and smoother commercialization. As manufacturing capabilities grow, the market can expand into new regions and treat more patients globally.
The biopharma sector is seeing a surge in investment and innovation, with both major global companies and emerging start-ups pouring resources into advanced therapies like cell, gene, and gene-modifying treatments. In 2018 alone, global investment in these advanced therapies reached about $13 billion. The following year, 19 major mergers and acquisitions in the cell and gene therapy (CGT) space were completed, totaling over $156 billion.
With more than 900 companies worldwide dedicated to CGTs and over 1,000 clinical trials in progress, the industry is poised for significant growth. Starting in 2025, we could see 10 to 20 new advanced therapies approved each year. Europe is a key player in this field, with 33% of these clinical trials taking place there.
Next-generation cell and gene therapies are making groundbreaking strides, offering potential cures for patients with few other treatment options, especially for rare and ultra-rare diseases. The primary focus areas for these therapies are cancer, musculoskeletal conditions, and eye diseases. Numerous products have already been approved and launched globally, and the number of clinical trials continues to increase.
In Europe, these therapies are classified as Advanced Therapeutic Medicinal Products (ATMPs). They are driven by a range of innovative scientific advancements, including CAR-T and TCR-T therapies, stem cells, siRNA, oligonucleotides, gene editing technologies (such as CRISPR, Zinc Fingers, and TALENs), and viral transfection methods.
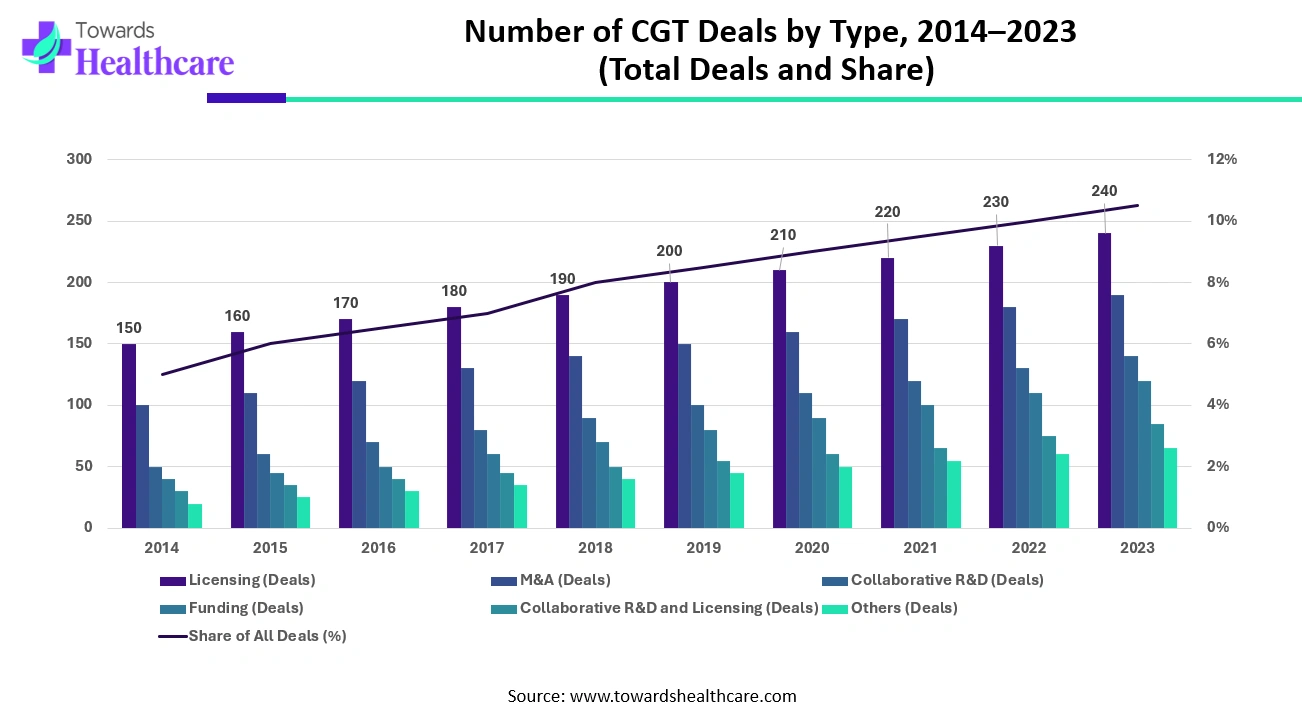
Collaborations, acquisitions, and investments play a crucial role in driving advancements and commercialization in the field of cell and gene therapy. The complex nature of these therapies requires multidisciplinary expertise and substantial financial resources, making strategic partnerships and investments essential for success. Here, we will explore the significance of collaborations, acquisitions, and investments in accelerating progress in the cell and gene therapy sector.
For instance,
Collaborations among pharmaceutical companies, biotech firms, academic institutions, and research organizations are common in the cell and gene therapy space. These collaborations bring together complementary expertise, resources, and technologies to tackle the challenges associated with the development, manufacturing, and commercialization of cell and gene therapies.
Strategic acquisitions are another means by which companies expand their capabilities and enhance their position in the cell and gene therapy market. Established pharmaceutical companies often acquire smaller biotech firms with promising pipelines or novel technologies. These acquisitions provide access to innovative therapies and specialized expertise, allowing larger companies to strengthen their product portfolio and accelerate the development and commercialization of new treatments.
Acquisitions in Cell & Gene Therapies in Q2 2021
| Acquisitions | Potential Deal Value (USD) |
| Sanofi Acquires Tidal Therapeutics | 47,00,00,000 |
| SparingVision Acquires GAMUT Therapeutics | Undisclosed |
| Athenex Acquires Kuur Therapeutics to Expand Cell Therapy Development with Off-the-Shelf Engineered CAR- NKT Platform | 18,50,00,000 |
| Charles River Laboratories to Acquire Vigene Biosciences to Enhance Gene Therapy Capabilities | 35,00,00,000 |
| Auris Medical Acquires RNA Therapeutics Company Trasir, Changes Name and Strategic Focus | Undisclosed |
| Brooklyn ImmunoTherapeutics Executes Letter of Intent to Acquire Novellus Therapeutics | 12,50,00,000 |
| Avalon GloboCare to Acquire SenlangBio in All Stock Transaction | Undisclosed |
| uniQure to Acquire Corlieve Therapeutics and Advance its Gene Therapy Program to Treat Temporal Lobe Epilepsy | 29,83,00,000 |
| Scopus BioPharma Expands Immunotherapy Pipeline with Acquisition of Olimmune | Undisclosed |
| Danaher Pays $9.6B for Aldevron, Manufacturer of High-Quality Plasmid DNA, mRNA, and Proteins | 9,60,00,00,000 |
In recent years, there has been a surge in partnerships, acquisitions, and investments in the cell and gene therapy space. Major pharmaceutical companies have been actively engaging in collaborations with biotech firms, academic institutions, and research organizations to gain access to promising technologies and therapies.
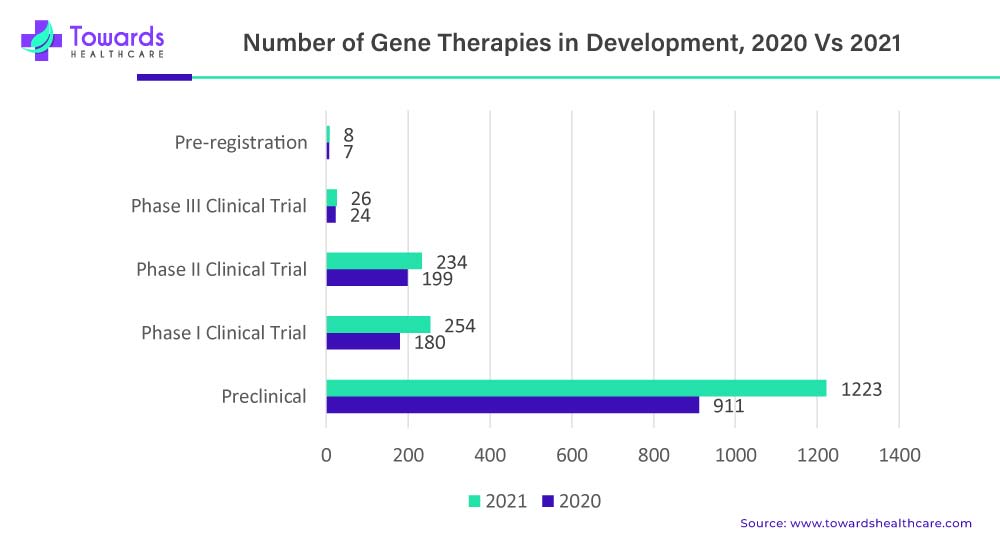
Clinical trials play a vital role in the development and commercialization of cell and gene therapies. These trials are essential for assessing the safety and efficacy of these innovative treatments, gathering evidence for regulatory approvals, and establishing their value in clinical practice. Here, we will explore the significant role of clinical trials in the cell and gene therapy market. In the comparison between 2020 and 2021, there was a notable rise in the number of therapies in preclinical development. In 2020, there were 911 therapies in preclinical studies, while in 2021, the number increased to 1,223 therapies. This indicates significant growth and focus on preclinical research and development in the field.
Clinical trials are designed to evaluate the safety profile of cell and gene therapies in human subjects. These trials follow rigorous protocols and monitoring procedures to identify any potential adverse effects or complications associated with the treatment. Safety data collected from clinical trials help researchers and regulatory authorities make informed decisions about the risks and benefits of these therapies. Clinical trials also assess the effectiveness of cell and gene therapies in treating specific diseases or conditions. As of May 2021, there were around 1,745 therapies in various stages of development, ranging from preclinical to preregistration. These trials measure various endpoints, such as improvements in patient outcomes, reduction in symptoms, or disease progression. Efficacy data generated from well-designed and controlled clinical trials are crucial for demonstrating the therapeutic benefits of these treatments.
Clinical trials focus on addressing unmet medical needs and providing innovative solutions for patients who have limited treatment options. By demonstrating the efficacy of cell and gene therapies in challenging diseases or conditions, clinical trials generate interest and demand for these treatments. This drives market growth by creating new opportunities to meet the needs of patients who were previously underserved. Clinical trials are instrumental in driving market growth in the field of cell and gene therapy. They provide evidence of safety, efficacy, and economic value, support regulatory approvals, and facilitate market access and reimbursement. Clinical trials also foster collaboration, expand the indications for these therapies, and address unmet medical needs. With the continued advancement of clinical trial programs, the cell and gene therapy market is expected to grow significantly in the coming years.
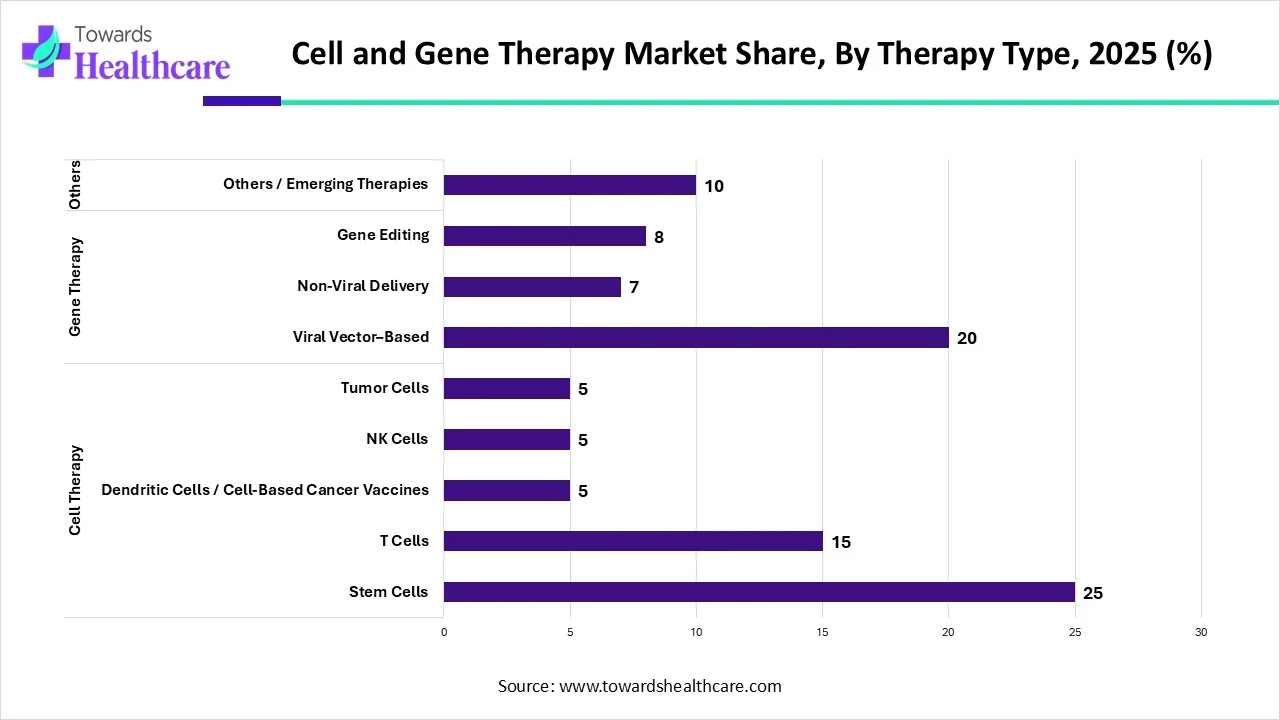
Which Therapy Type Segment Dominated the Cell and Gene Therapy Market?
By therapy type, the cell therapy segment registered its dominance by 55% over the global cell and gene therapy market. Cell therapy is the transplantation of human cells to replace or repair damaged tissues. The therapy is used to prevent or treat several chronic disorders. Stem cell-based therapies are one of the most common types of cell therapy used clinically. The segment’s growth is attributed to the growing research and development activities, technological advancements, innovative products, and the increasing number of clinical trials. As of November 2024, 21,746 cell therapy-based clinical trials have been registered in the clinicaltrials.gov.
Why Did the Infectious Disease Segment Dominate the Cell and Gene Therapy Market?
By therapeutic class, the infectious disease segment dominated the cell and gene therapy market in 2023. The rising incidences of infectious diseases and the COVID-19 pandemic increase the demand for cell and gene therapy for infectious diseases. The rising demand for genome editing methods like CRISPR/Cas for infectious diseases augments the market.
By therapeutic class, the oncology segment is anticipated to grow with the highest CAGR in the market during the studied years. The rising prevalence of cancer, the latest innovations, and favorable government initiatives to effectively treat cancer drive the segment’s growth.
| Delivery Method | 2025 Market Share (%) |
| In Vivo Delivery | 60 |
| Ex Vivo Delivery | 40 |
How the In Vivo Therapy Segment Dominated the Cell and Gene Therapy Market?
By delivery method, the in vivo therapy segment is expected to grow exponentially during the forecast period by 60%. In vivo, therapy involves the incorporation of cell and gene therapy products directly into the patient’s body instead of growing in a test tube. It is a simple and cost-effective method, eliminating the need to isolate cells from the patient, manipulate them, and insert them into the patient.
What Made Hospitals the Dominant Segment in the Cell and Gene Therapy Market?
By end-use, the hospitals segment held the largest share of the market in 2023. Hospitals provide all the necessary resources, including trained professionals, to provide appropriate cell and gene therapies.
The North American cell and gene therapy market was valued at US$ 1.2 billion in 2024, increased to US$ 1.3 billion in 2025, and is projected to reach approximately US$ 4.47 billion by 2034, growing at a CAGR of 14.05% from 2025 to 2034.
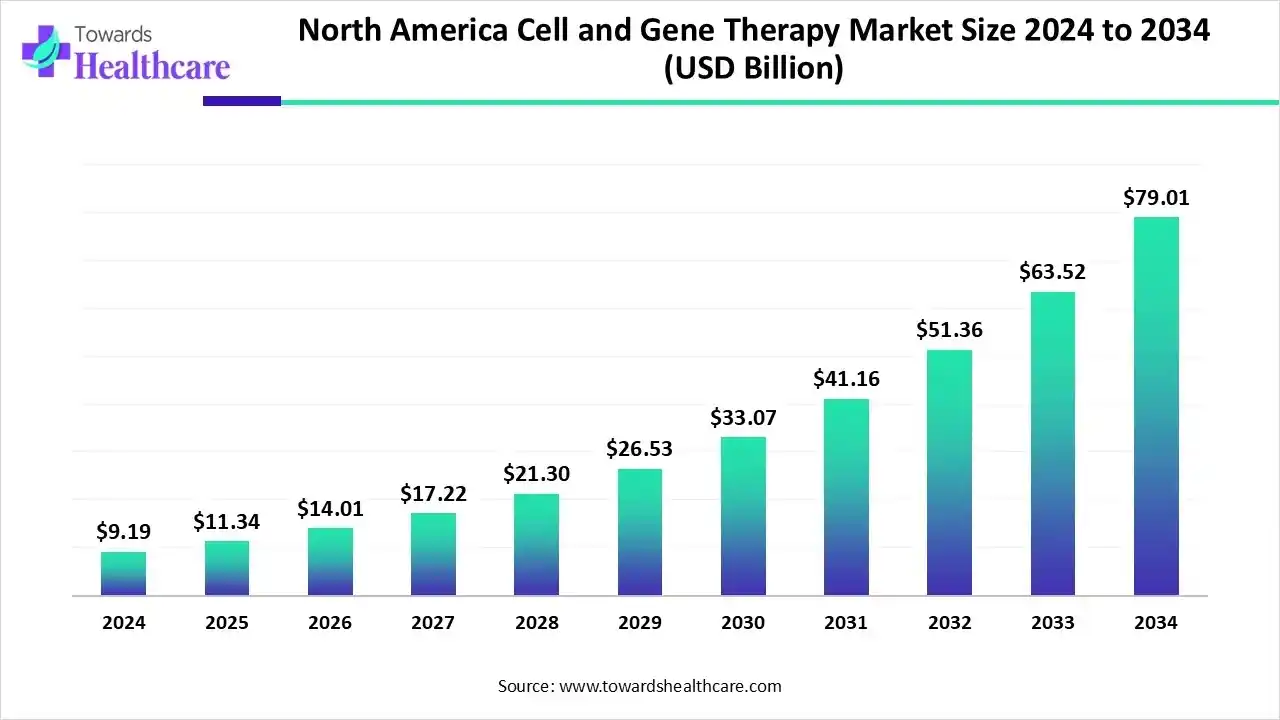
The North America region holds a prominent position in the global cell and gene therapy market. With its advanced healthcare infrastructure, strong regulatory framework, and significant investments in research and development, North America has become a hub for innovation and commercialization in this field.
The cell and gene therapy market in North America is experiencing significant growth due to the high prevalence of chronic diseases such as cancer, cardiovascular diseases, and genetic disorders. These conditions have created a demand for personalized and effective treatment options. Cell and gene therapies have emerged as promising solutions, addressing the root causes of these diseases and offering new possibilities for treatment and management.
The presence of leading pharmaceutical and biotechnology companies in North America further strengthens the market. These companies have been at the forefront of research and development in cell and gene therapies, investing heavily in clinical trials and infrastructure. Their expertise, combined with collaborations with academic institutions and research organizations, has accelerated the translation of scientific discoveries into commercial products. The regulatory landscape in North America has also been supportive of the cell and gene therapy market. The U.S. Food and Drug Administration (FDA) has established expedited regulatory pathways, such as the Regenerative Medicine Advanced Therapy (RMAT) designation and the Fast Track designation, to facilitate the development and approval of promising therapies.
The United States, in particular, dominates the cell and gene therapy market in North America. The country has a well-established biotechnology sector, a favorable regulatory environment, and a large patient population, making it an attractive market for cell and gene therapy companies. Several key players in the field are headquartered in the United States, driving innovation and market expansion. In January 2024, the Biden-Harris Administration announced that sickle cell disease would be the primary focus of the Cell and Gene Therapy (CGT) Access Model, owing to more than 100,000 cases in America. The main goal is to increase access to high-quality, affordable healthcare and lower healthcare costs.
The APAC cell and gene therapy market is valued at US$ 9.93 billion in 2024, increasing to US$ 5.71 billion in 2025, and is expected to reach approximately US$ 39.62 billion by 2034. This growth reflects a CAGR of 24.04% during the forecast period from 2025 to 2034.
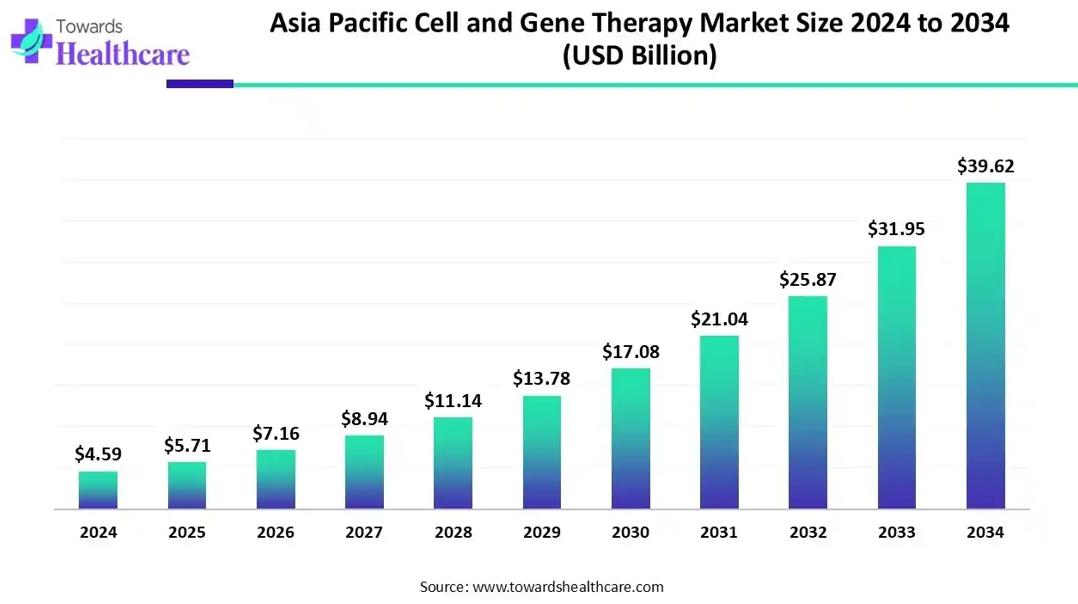
The Asia Pacific region is emerging as a promising market for cell and gene therapy. With its large and diverse population, increasing prevalence of chronic diseases, and growing investments in healthcare infrastructure, the region offers significant opportunities for the development and commercialization of cell and gene therapies.
Several countries in the Asia Pacific region have shown strong commitment to advancing cell and gene therapies through supportive regulatory frameworks and government initiatives. For example, Japan has implemented the Act on the Safety of Regenerative Medicine, which aims to accelerate the approval process for regenerative medicine products. China has also made significant investments in the field, launching the Made in China 2025 initiative to promote the development of innovative therapies.
The Middle East and Africa cell and gene therapy market is estimated at USD 1.15 billion in 2024 and grew to USD 1.41 billion in 2025. It is expected to reach approximately USD 9.75 billion by 2034, expanding at a CAGR of 23.85% from 2025 to 2034.
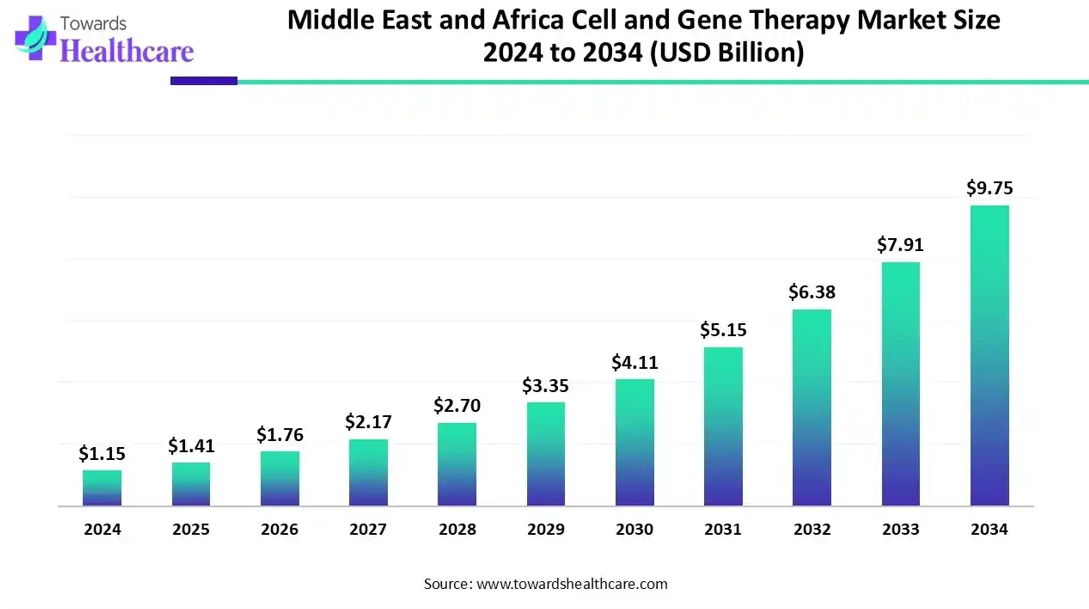
The UAE is home to numerous foreign companies, such as Charles River Laboratories and SGS SA, that manufacture CGT products to meet local demand. The UAE government also makes constant efforts to support CGT development and delivery to patients. In August 2024, the Department of Health Abu Dhabi provided gene transfer therapy for Duchenne muscular dystrophy for the first time in the emirate.
The Latin American cell and gene therapy market was valued at US$ 1.15 billion in 2024 and is expected to reach US$ 1.42 billion in 2025, with projections indicating it could approach nearly US$ 10.19 billion by 2034, growing at a CAGR of 24.40% over the forecast period.
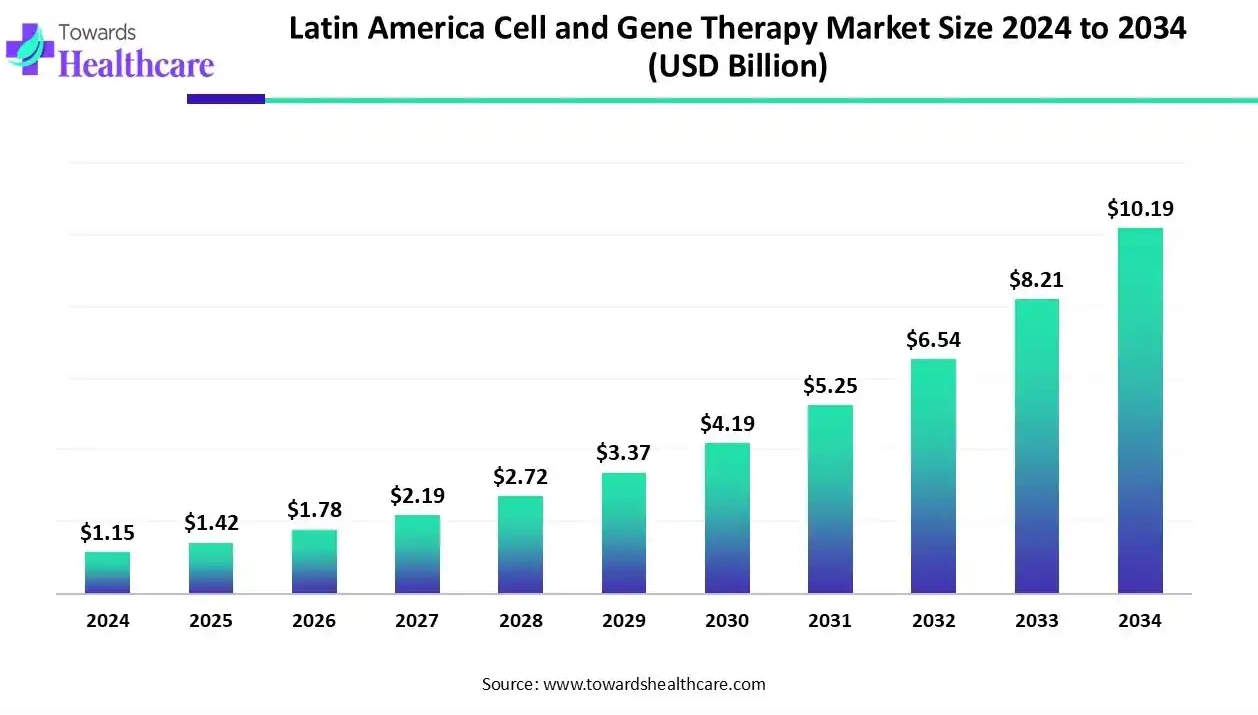
The Latin American cell and gene therapy market is experiencing strong growth, fueled by the rising prevalence of chronic and genetic diseases such as cancer, rare disorders, and immune conditions. Advances in biotechnology, gene-editing, and regenerative medicine are driving innovative therapies, while increased clinical trials in Brazil and Mexico, along with government support, investments, and strategic collaborations, are creating a favorable landscape. Growing awareness and adoption of personalized medicine are further boosting market potential across the region.
It is estimated that there are 186 biotech companies in Brazil. Brazilians are becoming more aware of CGT products and their benefits, necessitating their indigenous development. This demand is driven by the rising prevalence of chronic and genetic disorders. Approximately 13 million individuals are affected by a rare disease in Brazil. Also, the number of medical geneticists increased by 103% in a decade.
The Europe cell and gene therapy market size is estimated at US$ 5.74 billion in 2024, is projected to grow to US$ 7.17 billion in 2025, and is expected to reach around US$ 48.96 billion by 2034. The market is projected to expand at a CAGR of 23.90% between 2025 and 2034.
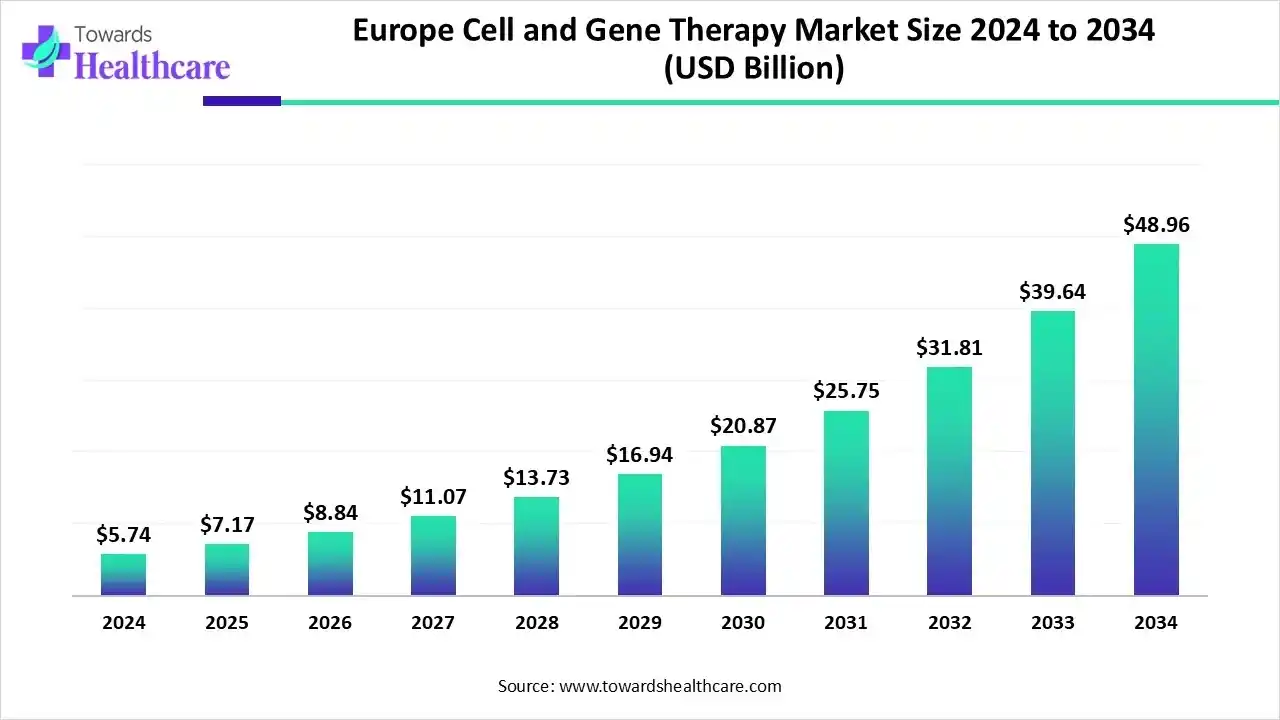
The Europe cell and gene therapy market is expanding due to increased government spending, supportive regulations, and rising demand for advanced therapies, with key players including biotech firms, pharmaceutical companies, and academic institutions. The growth is further fueled by the popularity of CAR-T cell therapies, backed by EMA approvals and funding initiatives like Horizon Europe.
The rapidly expanding research and clinical trial infrastructure propels market growth in the UK. As of 9th December 2025, around 56 clinical trials were registered in the UK related to clinicaltrials.gov website. The UK government has made several investments in the CGT field, including $10 million grant to the NHS Blood and Transplant and £17.9 million to the Advanced Therapy Treatment Center Network (ATCC Network).
Innovative therapies play a crucial role in the growth of the cell and gene therapy market. These therapies offer novel approaches to treating diseases and conditions by harnessing the power of cells and genes.
Innovative cell and gene therapies have the potential to address unmet medical needs for a wide range of diseases and conditions. They provide new treatment options for patients who have limited or no effective therapies available. By targeting diseases at the cellular and genetic levels, these therapies offer the potential for improved outcomes and quality of life for patients.
Cell and gene therapies have the ability to deliver personalized medicine approaches. They can be tailored to individual patients based on their genetic profile, disease characteristics, or other specific factors. Personalized therapies have the potential to be more targeted and effective, leading to better treatment outcomes. This shift towards personalized medicine is a significant driver for market growth. Innovative cell and gene therapies have shown promising results in the treatment of various types of cancers. For example, CAR T cell therapies have demonstrated remarkable success in certain forms of leukemia and lymphoma. These therapies involve modifying a patient's own immune cells to recognize and target cancer cells. Their potential to achieve long-lasting remission and even cure in some cases has revolutionized cancer treatment and contributed to market growth.
Innovations in gene editing technologies, such as CRISPR-Cas9, have opened up new possibilities for the treatment of genetic disorders. These technologies allow precise modification of genes, offering potential solutions for inherited diseases with a genetic component. Gene editing can correct genetic defects, restore normal gene function, or enhance therapeutic outcomes. The development and application of gene editing technologies have propelled the growth of the cell and gene therapy market. Regulatory agencies have recognized the potential of cell and gene therapies and have implemented expedited pathways to facilitate their development and approval. This regulatory support has encouraged investment in research and development, leading to the emergence of innovative therapies. Regulatory frameworks that prioritize patient safety while expediting the approval process have contributed to the growth of the market.
Leonard Schleifer, CEO of Regeneron, in a recent interview, suggested that the big thing for the biotech sector is gene therapy or genetics. He said that associating genes with disease is going to drive the pharmaceutical industry by emphasizing innovative treatments to repair certain genes and silence others.

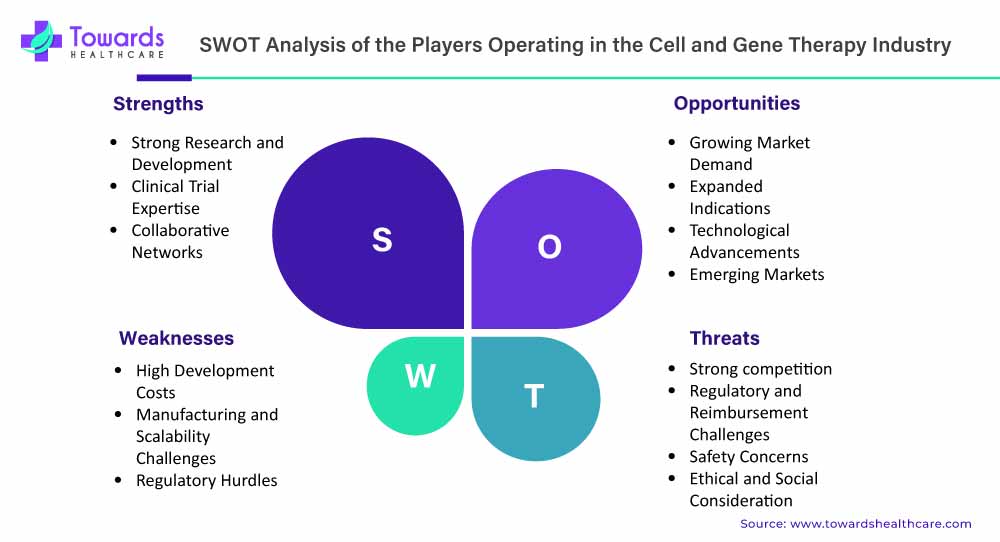
The cell and gene therapy market has experienced remarkable growth and progress in recent years, holding immense potential for transforming healthcare with personalized and targeted therapies. Several key players dominate the competitive landscape in this field. To gain a larger share of the market, players are using strategies like investments, alliances, acquisitions, and mergers.
Some of the major market companies include Alnylam Pharmaceuticals Inc., Amgen Inc., Biogen Inc., CORESTEM Inc., Dendreon Pharmaceuticals LLC., Helixmith Co. Ltd., JCR Pharmaceuticals Co. Ltd., Kolon TissueGene Inc., Novartis AG, and Pfizer Inc.
By Therapy Type
By Therapeutic Class
By Delivery Method
By End-Users
By Region
January 2026
January 2026
January 2026
January 2026