February 2026

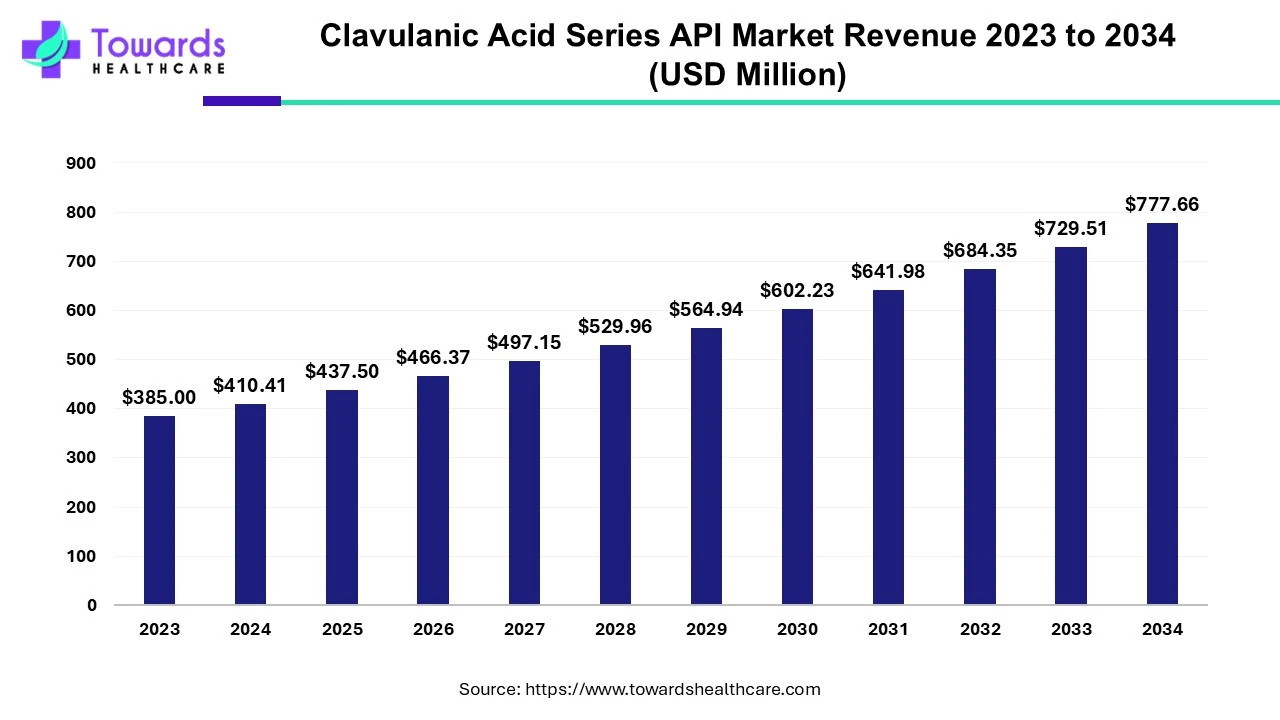
The clavulanic acid series API market was estimated at US$ 385 million in 2023 and is projected to grow to US$ 777.66 million by 2034, rising at a compound annual growth rate (CAGR) of 6.6% from 2024 to 2034. The rising incidences of antibiotic-resistant bacterial infections and growing research and development activities drive the market.
Clavulanic acid is a beta-lactam antibiotic used in conjunction with other antibiotics like Amoxicillin and Tircacillin to treat bacterial infections. Clavulanic acid is derived from the organism Streptomyces clavuligerus. Beta-lactamase is an enzyme that combats the effect of Amoxicillin in the body. Hence, clavulanic acid is a suicide inhibitor containing a beta-lactam ring in its structure that inhibits beta-lactamase, increasing the efficacy of Amoxicillin. However, clavulanic acid itself does not possess an antibiotic effect. The combination of clavulanic acid with Amoxicillin is called Augmentin. Clavulanic acid can significantly reduce the antibiotic resistance of antibiotics.
Augmentin is widely used to treat urinary tract infections (UTI), lower respiratory infections, sinusitis, otitis media, skin & skin structure infections, gynecologic infections, septicemia, and intraabdominal infections. Other off-label uses of Augmentin include animal bites, impetigo, COPD exacerbations, bronchiectasis, and odontogenic infections. The increasing incidences of beta-lactamase-producing bacterial infections promote the market.
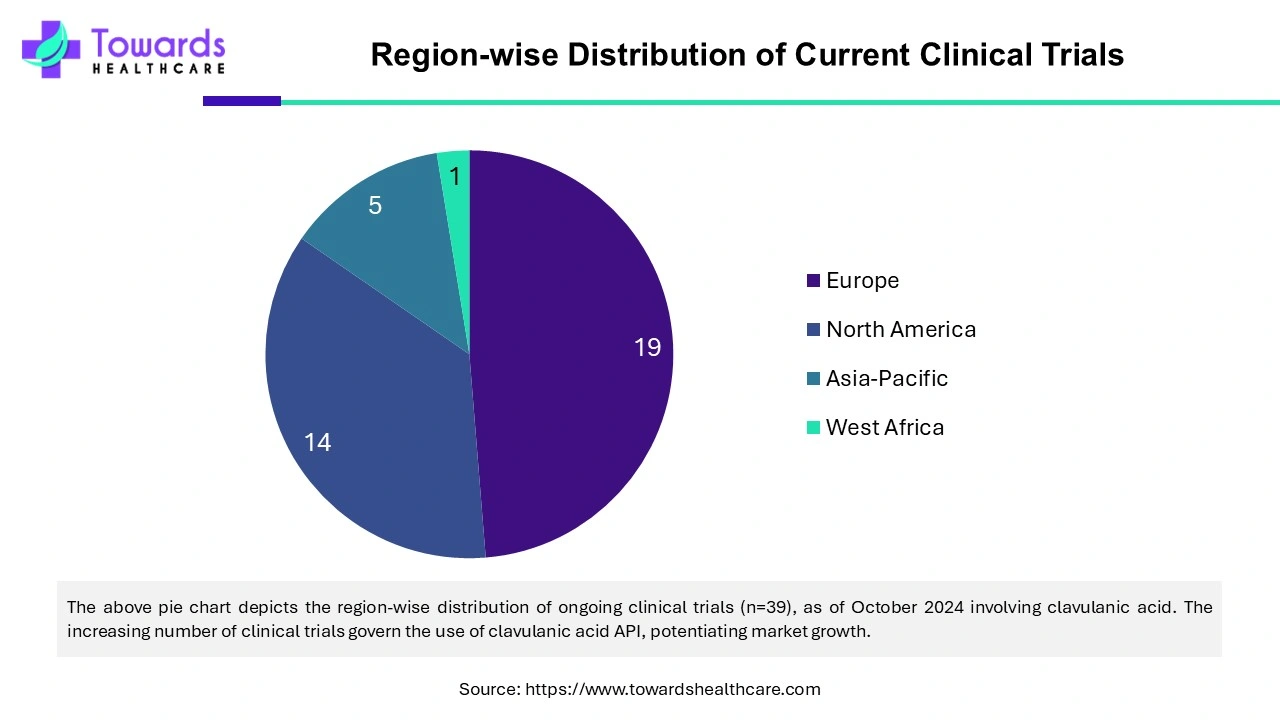
The significance of clavulanic acid is well-known among healthcare professionals. Several researchers are investigating the expanded use of clavulanic acid in combination with other drugs. Clavulanic acid has been studied for surgical site infections in pediatric uncomplicated appendectomy, dental infections, surgical site infections in colorectal surgery, etc. These effects of clavulanic acid are evaluated through preclinical and clinical studies. As of October 2024, 39 clinical trials are reported on clinicaltrials.gov involving clavulanic acid as an intervention. Out of these, 32 are recruiting, and seven are active but not recruiting. Clavulanic acid is evaluated for the treatment of cocaine use disorder, obstetric perineal tear, appendectomy, aspiration in ICU patients, etc. Thus, the growing demand for clavulanic acid and growing research & development for extended use results in more clinical trials, augmenting clavulanic acid series API market growth.
The preparation of the clavulanic acid series is very complex. The major challenge in producing clavulanic acid is the substrate availability. Clavulanic acid is produced from Streptomyces clavuligerus. Lack of substrate availability results in delayed production. Also the cost of the substrate is also high, limiting its affordability. ATCC sells the bacteria at a rate of $420 EA. Another major challenge is the downstream processing, i.e., recovery and purification processes.
North America dominated the clavulanic acid series API market in 2023. The state-of-the-art research & development activities, increasing bacterial infections, advanced healthcare infrastructure, and the presence of key players drive the market. The market is also governed by the increasing number of clinical trials in the region involving clavulanic acid. Out of the 39 ongoing clinical trials, 12 are being conducted in the U.S. and 2 in Canada. In 2022, Amoxicillin/clavulanic acid was the 96th most commonly prescribed medication in the US, accounting for more than 6 million prescriptions. According to the Centers for Disease Control and Prevention, flu was associated with 9.4 million illnesses and 4.3 million medical visits in the U.S. in 2022.
Asia-Pacific is anticipated to be the fastest-growing region in the clavulanic acid series API market during the forecast period. The state-of-the-art manufacturing facilities, increasing incidences of antibiotic-resistant bacterial infections, and increasing investments drive the market. China and India are the major producers of antibiotics globally. According to an IQVIA report, the sales of Augmentin in India jumped over 100% month-on-month, mopping up nearly Rs. 80 crore due to acute therapy, including respiratory and infectious disorders. The average Chinese production capacity of amoxicillin is 14,000 tonnes. China has an influenza hospitalization rate of 73 per 100,000 individuals, while in Hong Kong, the rate is 35.7 per 100,000 individuals. In 2022, around 13,202 influenza cases were reported in India.
Europe is expected to grow at a notable CAGR in the clavulanic acid series API market in the foreseeable future. The growing research and development activities and the rising adoption of advanced technologies bolster market growth. The rising incidence of bacterial infections and favorable government support propel the market. The growing emphasis on the indigenous manufacturing of the clavulanic acid series API. The presence of key players and increasing investments also contribute to market growth.
In France, 5,616 cases of Bordetella pertussis, or whooping cough, were recorded from January to May 2024. The number of infected people was higher than in previous outbreaks. The UK Health Security Agency (UKHSA) published an updated antimicrobial stewardship tool to support healthcare professionals across the UK to prescribe the most appropriate antibiotics for patients, while protecting their future effectiveness. Clavulanic Acid is classified as Access in the 2023 WHO AWaRe classification.
By type, the potassium clavulanate segment held a dominant presence in the market. This segment dominated because the clavulanic acid is available in its salt form with potassium. Potassium clavulanate gets activated after ingestion within the body to form clavulanic acid. It is a beta-lactamase inhibitor to improve the efficacy of antibiotics. It is always used in combination with several antibiotics prescribed for beta-lactamase-producing bacteria. The incidences of antibiotic resistance increase if the antibiotic is prescribed alone as it gets degraded by the enzyme. Hence, the rising incidences of antibiotic-resistant bacterial infections promote the use of potassium clavulanate.
By application, the oral medications segment accounted for a considerable share of the market. Clavulanic acid/Amoxicillin combinations are normally prescribed as oral tablets or capsules. Common brand names of Clavulanic acid/Amoxicillin combinations include Augmentin, Amoxiclav, or Clavulin. Oral medications are widely prescribed due to ease of administration and low cost, and they do not require any technical expertise for administration. The rising cases of flu, urinary tract infections, and other acute disorders augment the market.
By application, the injectable medications segment is projected to expand rapidly in the clavulanic acid series API market in the coming years. Injections are generally prescribed to pediatric and geriatric populations having difficulty in swallowing. Injectable medications are transported quickly throughout the body as they are directly administered in the body fluids and have 100% bioavailability. Clavulanic acid, in combination with other antibiotics, is given in injection before and after surgery or in hospitalized patients who are unable to swallow oral medications to reduce hospital-associated infections. The increasing number of hospitalizations and pediatric bacterial infections boost the market.
Anil Matai, Director General Organization of Pharmaceutical Producers of India (OPPI), commented that Penicillin G and Clavulanic Acid have been historically sourced primarily from China, leaving the Indian pharmaceutical sector vulnerable to external supply disruptions. The inauguration of production facilities will reduce the import dependence of these molecules by around 50%. (Source: Business Standard)
By Type
By Application
By Region
February 2026
January 2026
December 2025
November 2025