December 2025

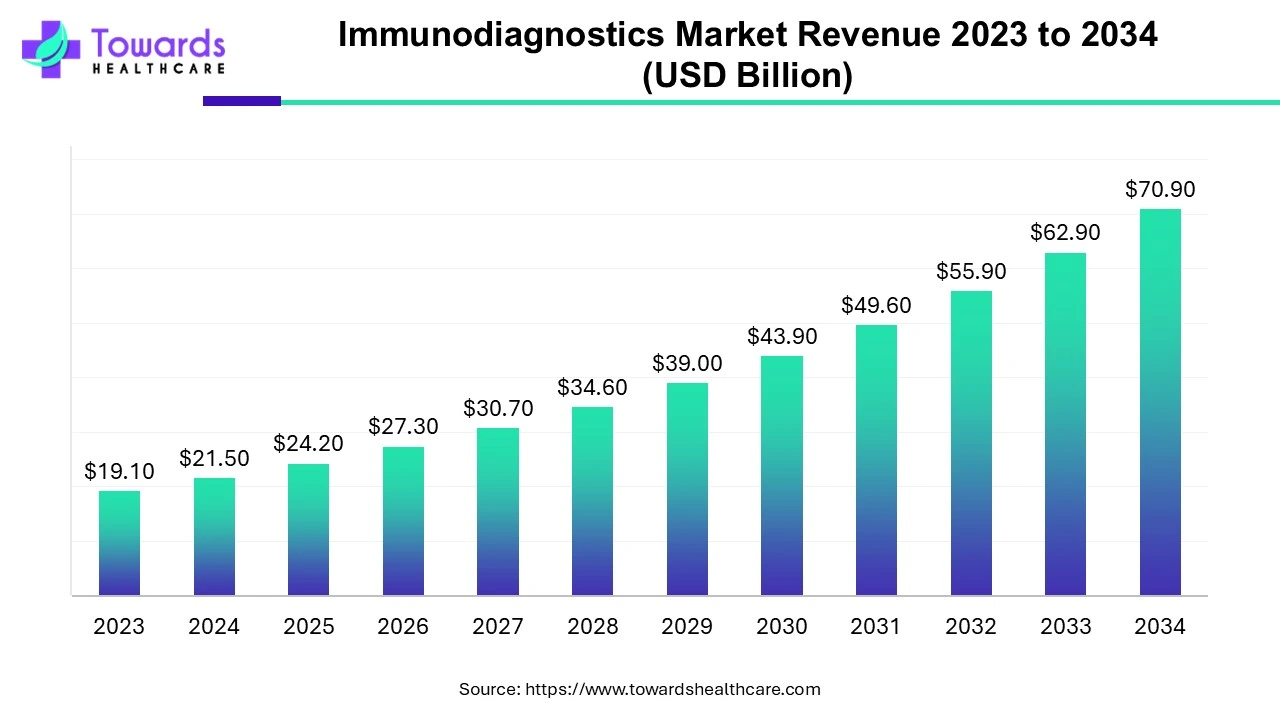
The global immunodiagnostics market was predicted at US$ 19.1 billion in 2023 and is projected to grow to US$ 70.9 billion by 2034, rising at a compound annual growth rate (CAGR) of 12.84% from 2024 to 2034. The widespread applications of immunodiagnostics techniques, including ELISA, radioimmunoassay, and lateral flow assays in the detection of infectious and autoimmune diseases, allergic conditions, and cancer, are contributing to the expansion of the immunodiagnostics market.
The immunodiagnostics market is growing significantly due to the tremendous innovations in the field of biotechnology through research and development of novel techniques like Enzyme-linked immunosorbent assay (ELISA), chemiluminescent immunoassays, and fluorescent immunoassays, etc. All of these diagnostics techniques are standardized for the diagnosis and detection of several types of diseases by identifying antigen-antibody interactions in human bodies. A variety of fluorescent chemical compounds, enzymes, substrates, reaction buffer components, and reaction chemicals are required for performing these test methods in hospitals, clinical research laboratories, and research institutes on a large scale.
The National Institute of Biologicals comprises an Immunodiagnostic Kit and Molecular Diagnostic Laboratory, which conducts quality control testing of molecular diagnostic test kits related to ELISA, RIA, CLIA, HIV, HBsAg, etc., that are ideal for blood donor screening of different viruses. The regulatory bodies responsible for allocating these test kits include the Central Drugs Standard Control Organization (CDSCO), the National AIDS Control Organization, and different government agencies. Moreover, Thermo Fisher Scientific Inc. is one of the leading providers of in-vitro diagnostic tests for allergy, asthma, and autoimmune diseases, with an annual revenue of $40 billion and more than 125,000 collaborations worldwide.
Several branches of immunology are expanding and are expected to reach massive progress in the coming years due to their specific applications in the determination of antigen-antibody interaction, microorganisms, autoantigens, allergens, alloantigens, and toxins. The major developments in immunodiagnostics include bead-based AlphaLISA, which reduces the duration and complexity of immunoassays, and microfluidic technologies, which enable the automated handling of process steps. The use of highly sensitive enzyme substrates, fluorescent and infra-red dyes, quantum dots, beads, gold nanoparticles, and nanocomposites led the immunodiagnostics market with precision and dynamics in test performances.
Standardization and quality assessment are important parameters in clinical laboratory analysis to obtain reliable clinical data sharing a similar degree of coherence. Several complement components and cytokines are extremely labile, which require specific pre-analytical handling, while immunoglobulins are stable at room temperatures for long hours. Immunoglobulins must be protected from precipitation and trapping in the blood clot during the entire pre-analytical stage to avoid false negative test results. Furthermore, the identification of risk factors with the help of genetic biomarkers to control the transmission of diseases showcases another challenging factor in front of the researchers.
North America dominated the immunodiagnostics market in 2023 due to a large number of elderly population, the growing incidence of chronic and infectious diseases, and the expansion of immunodiagnostics tests for early disease management. The National Center for Immunization and Respiratory Diseases comprises the divisions of bacterial and viral diseases to prevent and control vaccine-preventable health conditions caused by bacteria and viruses. There is also an immunization services division of the NCIRD in the U.S. that provides funding for immunization information systems and arranges research programs, training, and education activities for children’s vaccination. NCIRD is also equipped with an influenza division for the global prevention and control of novel influenza viruses, as well as the coronavirus and other respiratory viruses division, which works to improve public health.
The International Union of Immunology Societies (IUIS) established a Quality Assessment and Standardization (QAS) Committee in immunology to handle the standardization challenges in immunology testing.
The Quality Assessment and Standardization (QAS) Committee works with the Allergen Standardization Subcommittee, the Autoantibodies in Rheumatic and Related Diseases Subcommittee, the Complement Subcommittee, the Leukocytes Subcommittee, and the Big Data in Immunology Subcommittee to operate the rising Challenges in Immunology testing and clinical research laboratories.
Asia Pacific is the fastest-growing region in the immunodiagnostics market due to the progress in healthcare infrastructure, the rising healthcare expenditure, the increasing health consciousness, and the growing investments by leading industrial players. This region offers new product approvals and appropriate reimbursement plans for products and tests. Itovebi, a product of Roche, recently received USFDA approval based on clinical data reporting a reduction of more than 50% death risks from advanced-stage or hard-to-treat breast cancer. Roche Diagnostics in Singapore offers two blood screening technologies: molecular testing using polymerase chain reaction technology and serological testing using electrochemiluminescence technology. The advancements in personalized medicines and clinical biomarkers are offering clinicians benefits in identifying infectious pathogens. The Government of India established the National Centre for Disease Control and the National Institute of Communicable Diseases to organize and expand the activities to control communicable diseases.
By product, the reagents and consumables segment held the largest immunodiagnostics market share due to their high demand for manufacturing, sales, distribution, and single-use products. QIAGEN or Thermo Fisher Scientific Inc. are the leading providers of reliable and high-quality laboratory-grade products, which include reaction buffers, reagents, chemicals, enzymes, substrates, reaction dyes, etc. These products offer long-term storage capacity with useful features of optimum pH, light sensitivity, optimum temperature conditions, etc., suitable for test performance.
By technology, the enzyme-linked immunosorbent assay segment led the immunodiagnostics market remarkably due to its ideal properties for the detection of COVID-19 pandemic cases, and accurate test results with the help of enzymes and related reaction components. A colored product formed by antigen-antibody interaction in this technique gives an exact idea about the presence or absence of disease in the human body.
By application, the oncology and endocrinology segment led the immunodiagnostics market due to the emergence of high-performance testing methods and innovative drug solutions. With the rising shift towards technologies like artificial intelligence and quantum computing, researchers are able to provide personalized cancer treatments and medicines to patients. Endocrine diseases are also identified and treated by using molecular and immunological assays inside the laboratories.
By end-user, the clinical laboratories segment is estimated to grow at the fastest rate during the forecast period due to the rising shift of a large patient population from hospitals and testing laboratories. Patients are able to get immediate test results by going through blood screening tests which provide them with accurate treatment plans and medications from doctors or consulting firms.

By Product
By Technology
By Application
By End User
By Region
December 2025
December 2025
November 2025
November 2025