February 2026

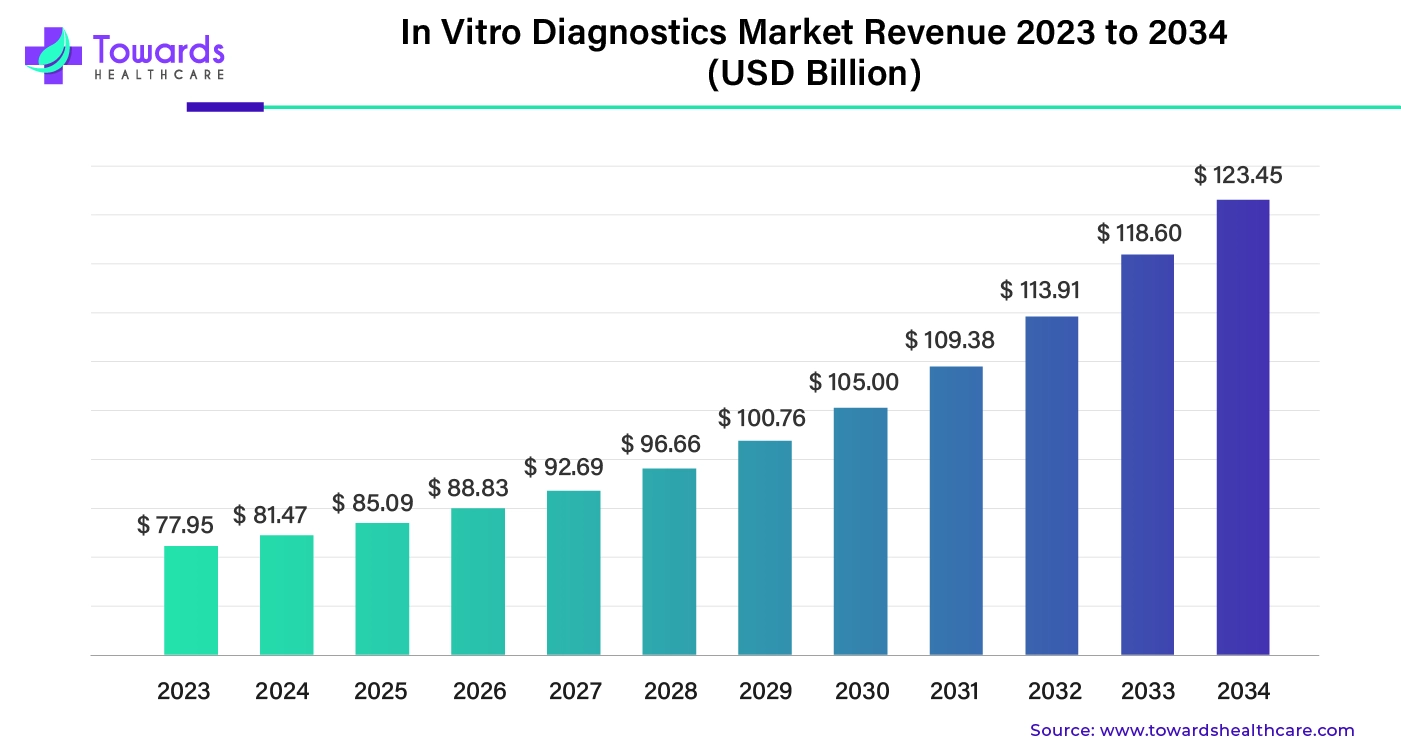
The global in vitro diagnostics market was estimated at US$ 77.95 billion in 2023 and is projected to grow to US$ 123.45 billion by 2034, rising at a compound annual growth rate (CAGR) of 4.45% from 2024 to 2034.
| Metric | Details |
| Market Size in 2024 | USD 81.47 Billion |
| Projected Market Size in 2034 | USD 123.45 Billion |
| CAGR (2025 - 2034) | 4.45% |
| Leading Region | North America |
| Market Segmentation | By Product, By Technology, By Application, By End-use, By Region |
| Top Key Players | BD, F. Hoffmann-La Roche Ltd., Qiagen, Siemens Healthineers AG, Agilent Technologies, Inc., Bio-Rad Laboratories, Inc., QuidelOrtho Corporation, Charles River Laboratories, Abbott, Danaher Corporation, bioMérieux SA, Quest Diagnostics Incorporated, Sysmex Corporation |
According to the World Health Organization, in vitro diagnostics (IVDs) are different types of tests conducted for the detection of infections, diseases, and conditions. IVDs are conducted using glass equipment in the laboratory, which is completely different from in vivo tests, which are conducted in the body itself. The in vitro diagnostics market is involved in the production of kits & reagents, biological material, equipment, and other resources needed for conducting tests. Various other aspects associated with IVD, such as conducting evidence-based research, clinical trials, gathering data, etc, are also part of the market. The growing prevalence of various diseases and the need to control the spread of the disease increases the demand for in vitro diagnosis.
AI and machine learning have become an essential part of the healthcare industry. Smart diagnostics has become a thing due to the integration of AI in IVDs. AI plays a significant role in clinical decision-making because healthcare professionals can rely on the judgment made by AI algorithms. AI and machine learning have been integrated into various diagnostic equipment, software, and applications. This addition helps in analyzing large data, providing accurate results, reducing time & cost, conducting predictive analysis, decision-making, and much more. With the growing burden of healthcare professionals and resources, AI can be highly useful in managing resources, efforts, and time, which can lead to cost savings and staff satisfaction.
For instance,
The United Nations developed 17 sustainable development goals (SDGs), which focus on the sustainable development of different fields. Among the 17 SDGs, SDG 3 is ‘Good Health and Well-Being,’ which significantly helps in the growth of the market. One of the targets of SDG 3 is reducing the cases of tuberculosis, AIDS, and malaria, combatting hepatitis and neglected tropical diseases, and combating water-borne diseases and other communicable diseases. This goal significantly requires in vitro diagnostics resources and tools. Another component of the goal is to promote R&D on medicines and vaccines for communicable and non-communicable diseases.
Technological advancements have improved IVDs. Now, it is possible to get accurate results in less time. However, this has also become a restraining factor for the growth of the in vitro diagnostics market because these advanced diagnostics are expensive, and a lot of people cannot afford them, especially those who belong to the lower and middle classes. In countries where even basic healthcare facilities are not available, advanced diagnostic resources are not accessible. Apart from this, even in developed countries, it becomes difficult to provide these resources in rural and remote areas.
A lot of efforts have been made to grow the in vitro diagnostics market in recent years. Point of care holds great potential for improving IVDs. Point-of-care testing (POCT) means conducting a diagnosis wherever the patient is located. This allows the market players to visit people in remote locations to provide different diagnostic services. Currently, there are various tests and devices available for POCT, and in the future, there is scope for developing highly sensitive, rapid, and low-cost tests.
For instance,
By product, the reagents segment dominated the in vitro diagnostics market in 2023 and is expected to remain dominant in the predicted time frame. This segment consists of raw materials, kits, solutions, enzymes, primers, antigens, antibodies, buffers, and other components that are needed for conducting tests. These components come in the form of kits or sold individually based on their needs and demand. The reagents are clinically approved and undergo rigorous monitoring and quality control, which ensure that the end products are of top quality and provide accurate results when used. Reagents are the most important part of IVDs, and hence, this segment is growing rapidly.
For instance,
By technology, the immunoassay segment held the largest share of the in vitro diagnostics market in 2023. It is estimated that more than 100 million immunoassay tests are conducted every year. Immunoassays are widely used in various important areas of pharmaceutical analysis for disease diagnosis, therapeutic drug monitoring, and drug discovery. Immunoassays have high throughput, high sensitivity, and inherent specificity. Immunoassays are used in in monitoring allergic agents, infectious diseases, proteins, hormones, antibodies, TDM, and drug abuse.
For instance,
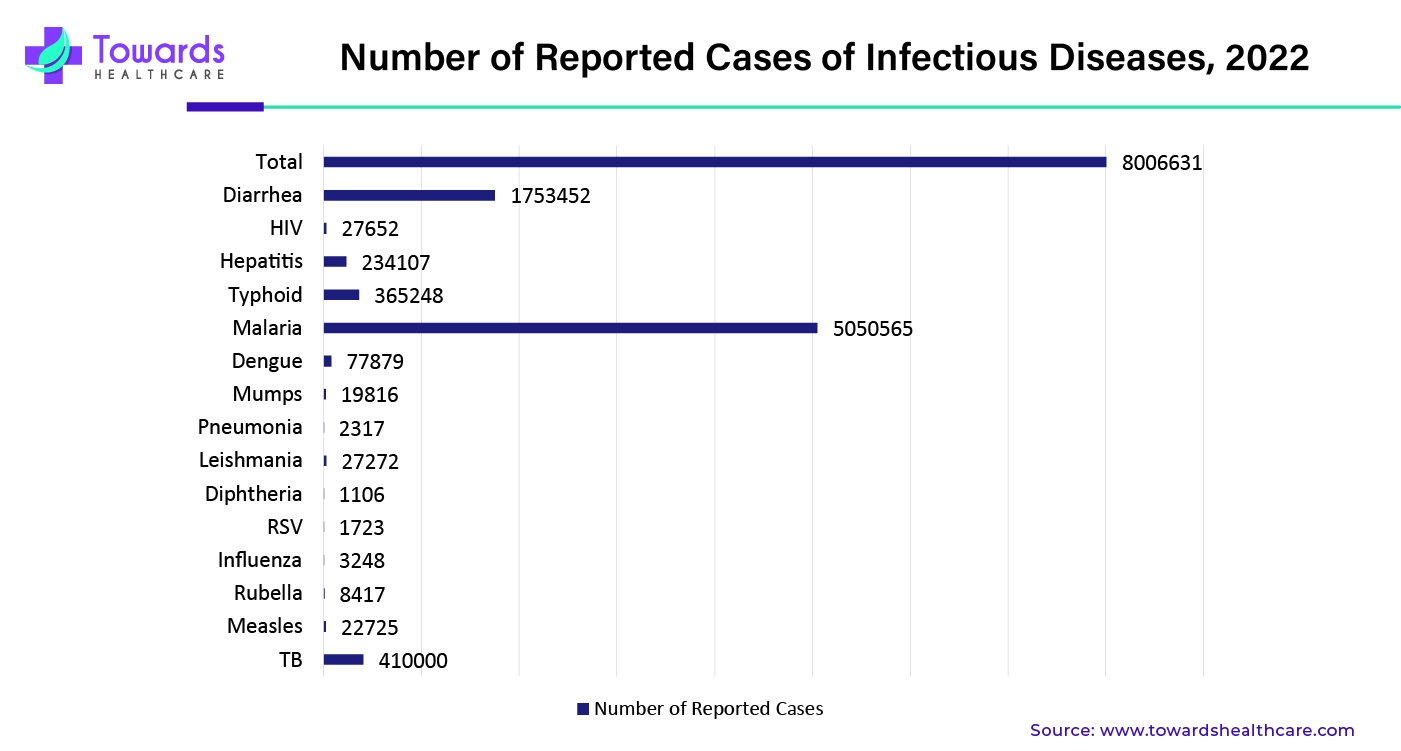
The infographic represents the total cases of infectious diseases along with the prevalence of different infectious diseases. From the graph, it can predicted that the major contributors to the growth of the in vitro diagnostics market are malaria, HIV/AIDS, TB, diarrhea, typhoid, and hepatitis.
By application, the infectious diseases segment held the largest share of the in vitro diagnostics market in 2023. Infectious diseases are contagious and, hence, require continuous diagnosis. The COVID-19 pandemic is one of the biggest examples of infectious diseases and the importance of diagnosis. In vitro diagnostics is an essential component of controlling the spread of infectious diseases, as they can be life-threatening if not treated on time. Timely diagnosis also prevents its spread and reduces the burden of healthcare costs. According to the World Health Organization, lower respiratory infections are the 5th leading cause of death, and tuberculosis is the 10th leading cause of death globally.
By end-use, the hospitals segment was dominant in the in vitro diagnostics market in 2023. With a large number of patient visits, the segment dominates in IVDs. On a daily basis, various IVD tests are run to diagnose diseases and monitor patients' health. Hospitals have their own laboratories for conducting tests and are equipped with advanced technologies. Patients prefer listing hospitals due to the advanced infrastructure and availability of healthcare professionals for different needs. Many patients, especially elderly patients who need continuous monitoring, are obligated to visit and stay in hospitals, which increases the demand for hospitals.
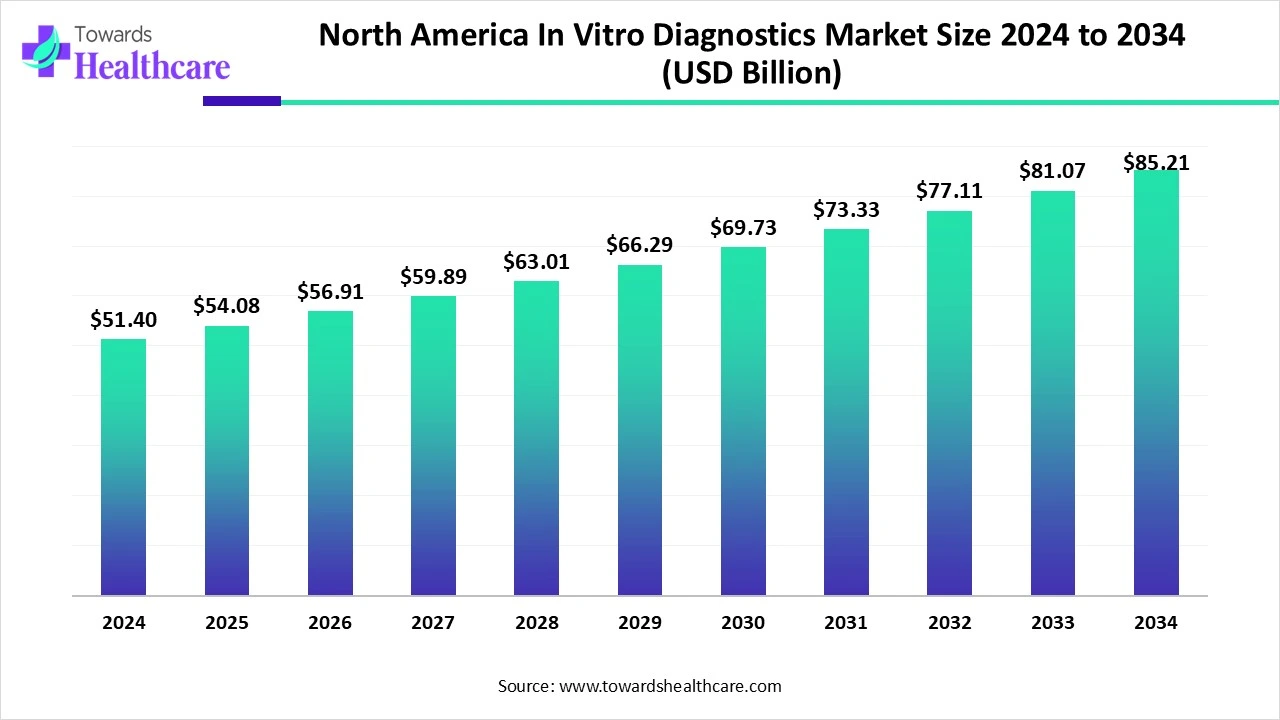
The North American in-vitro diagnostics market size is valued at $51.4 billion in 2024 and is expected to grow to $54.08 billion by 2025. Looking ahead, it's projected to reach around $85.21 billion by 2034, growing at an average annual rate of 5.23% from 2025 to 2034.
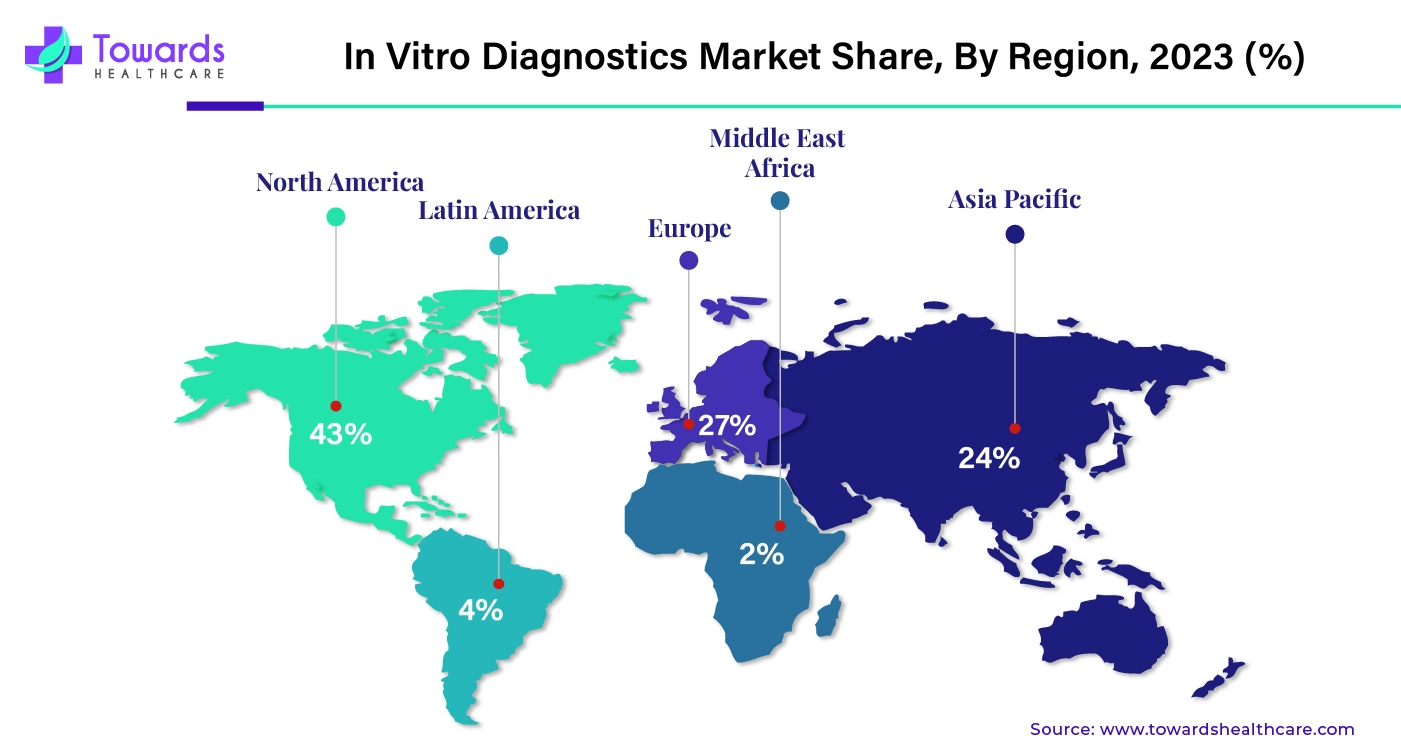
By region, North America dominated the in vitro diagnostics market share by 43% in 2023. The growth of the market in North America is attributed to the advanced healthcare system. The hospitals, clinical laboratories, and other healthcare infrastructure are equipped with advanced equipment and diagnostics resources. Another factor that contributes to the growth of the market in this region is the presence of key market players that contribute to developing new products and services in IVDs. North America also holds the largest share of the pharmaceutical and biotechnology industries, which significantly contributes to IVDs. The U.S., Canada, and Mexico are the major contributors in the revenue generation.
The U.S. held the dominant share of the in vitro diagnostics market in the North American region due to advanced infrastructure, highly qualified healthcare professionals, and growing awareness about IVDs. In 2022, the spending on the U.S. healthcare system increased by 4.1%, which was equivalent to US$ 4.5 trillion or US$ 13,493 per person. Another factor that contributes to the dominance is the growing prevalence of various chronic communicable and non-communicable diseases. Some of the major health issues that need IVD are tuberculosis (8331 cases in 2023), chronic lower respiratory disease (More than 35 million people live with this condition), and cancer (1,958,310 new cancer cases in 2023).
Canada is also among the countries with an advanced healthcare system. The Government of Canada constantly makes efforts to develop the healthcare infrastructure. For instance, to strengthen public healthcare for over ten years, the government invested US$ 200 billion. To tackle supply shortages of medical devices and essential drugs, the government also invested US$ 3.2 million.
The Asia Pacific in vitro diagnostics market is estimated to be worth $21.8 billion in 2024 and is expected to grow to $23.25 billion in 2025. Looking ahead, the market could reach around $41.46 billion by 2034, growing at an average annual rate of 6.64% from 2025 to 2034.
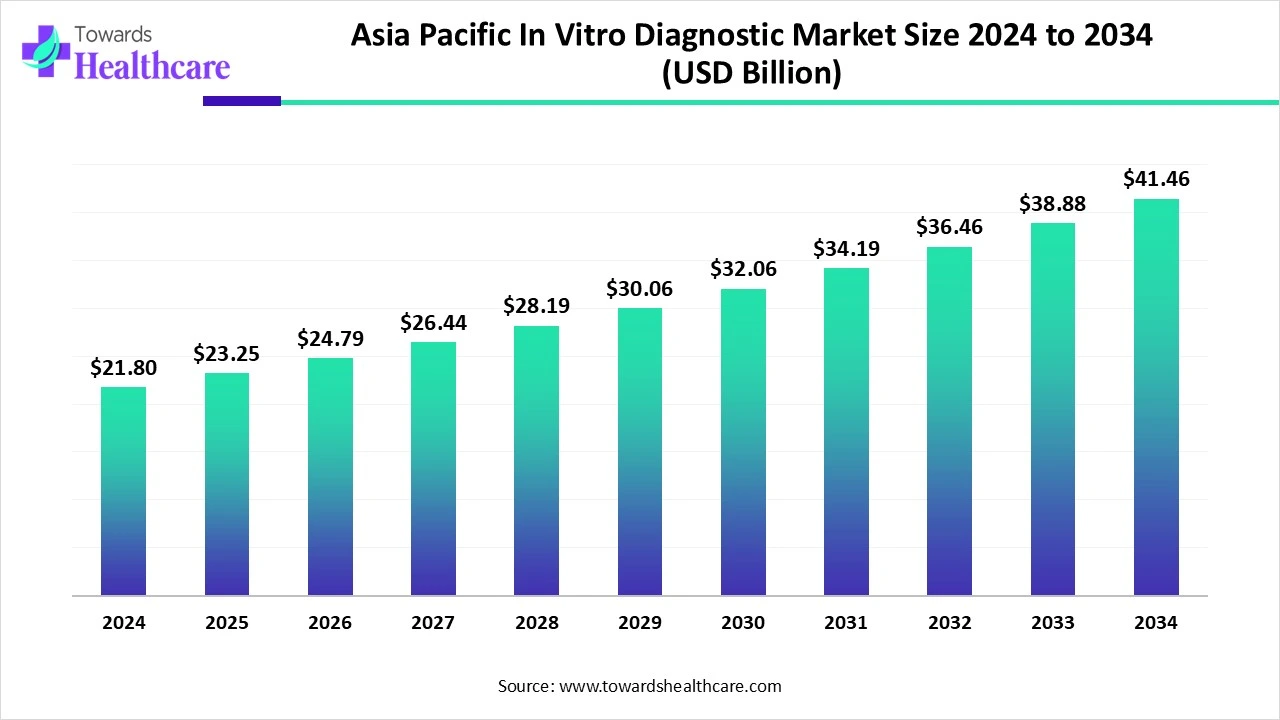
By region, Asia Pacific is expected to grow at a significant rate during the forecast period. The growing prevalence of diseases is driving the in vitro diagnostics market. Lack of education, lack of healthcare services in rural areas, and growing population contribute to the growing number of diseases in Asia Pacific. The governments in Asia Pacific are continuously making efforts to tackle this challenge. The market is also growing because many market players are taking initiatives to provide resources that will improve the healthcare systems, especially in underdeveloped and developing countries.
India is contributing to the growth of the in vitro diagnostics market due to the growing prevalence of diseases. There is a growing number of infectious diseases and chronic conditions in the country. The Government of India is taking various initiatives to tackle the challenges. Of the initiatives is the Infectious Disease Biology Program, which aims to provide solutions to various diseases, including vector-borne diseases, HIV/AIDS, and tuberculosis. The healthcare budget for 2024-2025 also indicated the seriousness of addressing health issues in India. The budget for 2024-2025 was Rs. 90,958.63 crore, which is 12.9% more than the healthcare budget for 2023-2024, which was Rs. 87,656.90 crore.
For instance,
The Europe in-vitro diagnostics market size is valued at $27.5 billion in 2024 and is expected to grow to $29.13 billion by 2025. By 2034, the market is projected to reach around $48.92 billion, expanding at an average annual growth rate of 5.93% between 2025 and 2034.
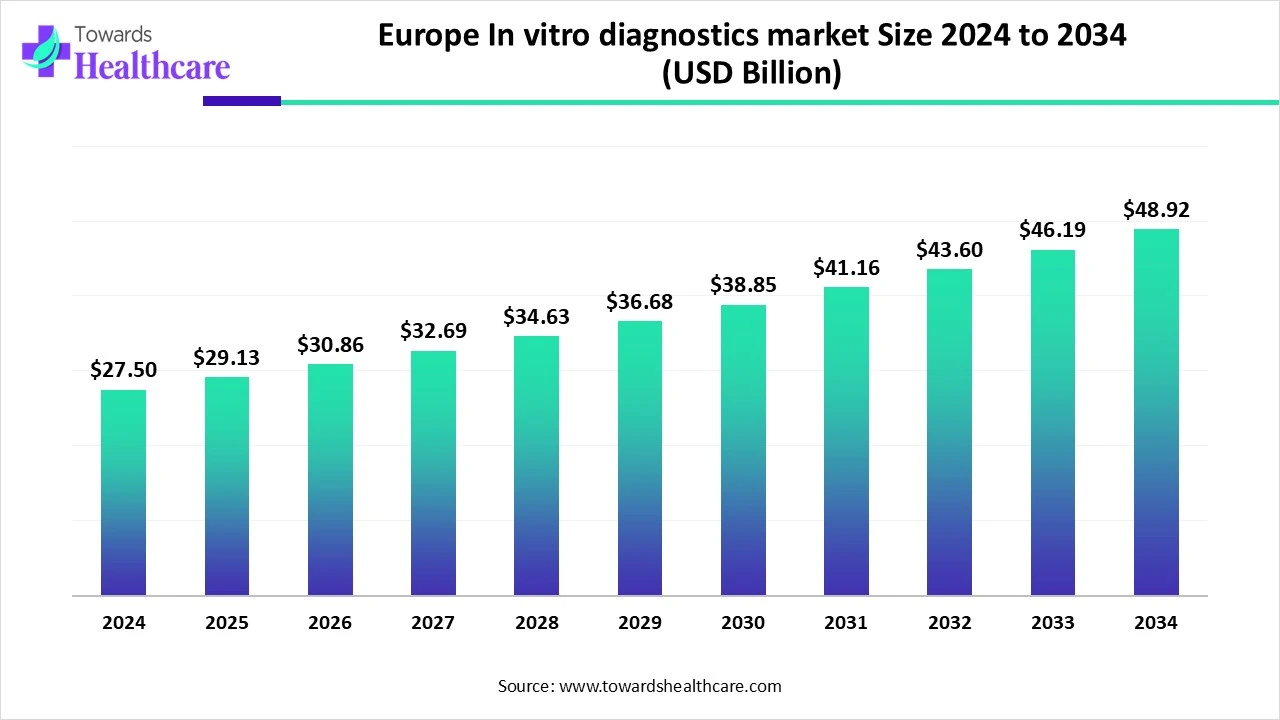
Europe is estimated to grow significantly during the forecast period. Well-established infrastructure, rising healthcare costs, and the frequency of cancer, infectious diseases, and other illnesses are some of the reasons for the rise. This will probably encourage the use of these tools, especially when combined with the increasing number of POC device introductions.
In 2024, Germany controlled the European market. The country's in-vitro diagnostics market is expanding due to a number of factors, including the rising incidence of chronic diseases like diabetes and cancer, a well-established healthcare system, increased diagnosis rates, and the growing use of new tests and instruments based on cutting-edge methods by healthcare facilities.
Latin America is expected to grow significantly in the In Vitro Diagnostics market during the forecast period. Latin America is experiencing a rise in the incidence rates of infectious diseases. This is increasing the demand for in vitro diagnostic devices for their early and fast detection. At the same time, advanced technologies are being used for the development of molecular diagnostics and to accelerate their development. Additionally, growing home care is also increasing its use. Moreover, the growing research and development are leading to the development of personalized diagnostic kits. These developments are being supported by the funding and investment provided by the government. Thus, this is promoting the market growth.
In August 2025, bioMérieux, the leader in the field of in vitro diagnostics, along with Ocean Spray, launched GENE-UP® PRO HRM, where the global head of the xPRO™ Program at bioMérieux, Ben Pascal, stated that they are always ready to overcome the spoilage problems as they are a longstanding diagnostic partner of the beverage industry. Thus, a custom microbiology quality control test was developed, which showed a wide range of implications for all concentrates and juices manufacturers by collaborating with Ocean Spray.
Stephane Argivier, CEO of Logical Biological, commented on his appointment as the new CEO of the company that he could see great potential in the company due to their deep expertise, quality, and exceptional customer service to deliver critical and complex bioreagent solutions to the IVD market. He envisions to expand the business further and deliver value to both the customers and shareholders.

In November 2025, Agilent Technologies Inc. released its Q4 2024 Earnings Call, where a 0.8% revenue growth of $1.701 billion was reported. Similarly, $426 million was observed, indicating a total of 5% growth in the Agilent crosslab group revenue. Additionally, the operating margin was reported to be 27.4%. Similarly, 6% year-over-year, with $1.46 for earnings per share (EPS) was also noted in the report.
$481 million and $1.75 billion were reported for operating cash flow for the quarter and the year, respectively. Moreover, 6.79 billion to $6.87 billion, reflecting 4.3% to 5.5% growth, was observed in fiscal year 2025 revenue guidance. The fiscal year 2025 EPS guidance showed a rise of 5% to 6% with $5.54 to $5.61 growth. Additionally, a growth of $1.65 billion to $1.68 billion and $1.25 to $1.28 was reported for Q1 2025 revenue guidance and Q1 2025 EPS guidance, respectively.
In October 2024, financial results for the third quarter ended Sept. 30, 2024, were released by Abbott. $0.94 and $1.21 were noted for GAAP diluted EPS and adjusted diluted EPS, respectively, for the third quarter. The organic sales showed a rise with a range of 9.5% to 10.0%. Additionally, a growth at the midpoint of the guidance range was observed by the company with $3.34 to $3.40 for full-year diluted EPS on a GAAP basis and $4.64 to $4.70 for adjusted diluted EPS.
| Company Name | F. Hoffmann-La Roche Ltd. |
| Headquarters | Basel, Switzerland, Europe |
| Recent Development | In September 2024, with the addition of over 20 cutting-edge artificial intelligence (AI) algorithms from eight new partners, Roche announced the expansion of its digital pathology open environment. Using state-of-the-art AI technology, these strategic partnerships seek to assist scientists and pathologists in the study and detection of cancer. |
| Company Name | Siemens Healthineers AG |
| Headquarters | Erlangen, Germany, Europe |
| Contribution |
The company is a global provider of various healthcare products, including diagnostic products and services. In the year 2023, the company’s diagnostics segment revenue was € 36 billion. In August 2023, Medical Physics Holdings, LLC's membership interest was fully bought by Siemens Healthineers. Cash payments of US$94 million (€85 million at the time of acquisition) were made for the transaction. Separate from the sale, Siemens Healthineers redeemed Aspekt Solutions' financial obligations, which totaled US$ 12 million (€11 million at the time of acquisition). |
By Product
By Technology
By Application
By End-use
By Region
February 2026
January 2026
January 2026
January 2026