January 2026

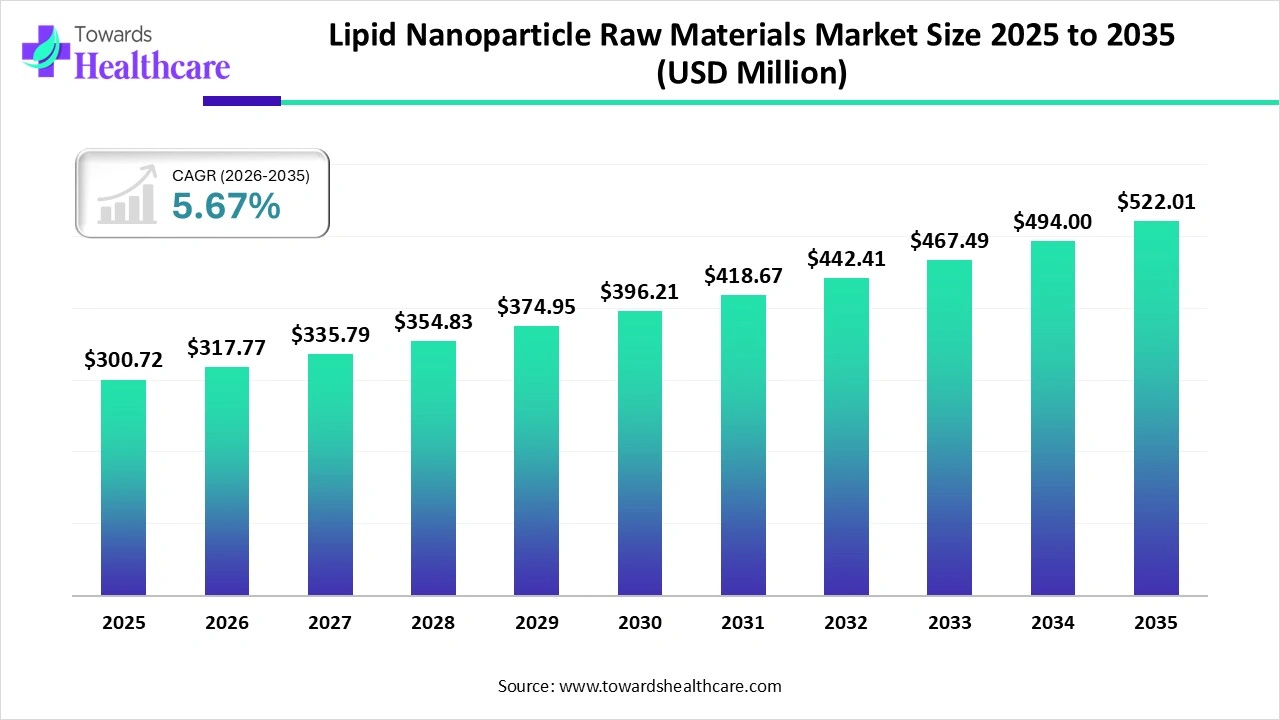
The global lipid nanoparticle raw materials market size is projected to reach USD 522.01 million by 2035, expanding from USD 300.72 million in 2025, at an annual growth rate of 5.67% during the forecast period from 2025 to 2035.
| Key Elements | Scope |
| Market Size in 2025 | USD 300.72 million |
| Projected Market Size in 2035 | USD 522.01 million |
| CAGR (2026 - 2035) | 5.67% |
| Leading Region | North America by 41% |
| Market Segmentation | By Product, By Disease Indication, By Application, By Region |
| Top Key Players | Polysciences, Inc., NOF AMERICA CORPORATION, Biopharma PEG Scientific Inc., Creative Biolabs, CordenPharma International, Tebubio, Avanti Polar Lipids, Hopewell Therapeutics, Echelon Biosciences, Merck KGaA, BroadPharm, Cytiva |
The lipid nanoparticle raw materials market deals with the production and distribution of raw materials used in the development of lipid nanoparticles. The four main components of lipophilic nanoparticles (LNPs) are cationic or ionizable lipids, which bind to negatively charged biological material and facilitate endosomal escape; phospholipids, which give the particle structure; cholesterol, which facilitates membrane fusion and adds stability to the nanoparticles; and pegylated lipids, which enhance the stability and circulation of the nanoparticles.
Lipid-based nanoparticles are known as LNPs. A unique pharmaceutical formulation and a novel pharmaceutical drug delivery system are what they are. The discovery of Doxil®, the first small-molecule liposomal preparation to get FDA approval in 1995, marked a turning point in the study of lipid-based nanocarriers, which is now progressing quickly in the fields of biologics delivery and cancer treatment. The pharmaceutical industry has seen a rise in the use of LNPs as potential delivery systems for a range of medicines.
Lipid nanoparticles have several advantages over many delivery systems used in therapeutics, which increases the usage of LNPs and, hence, the growth of the lipid nanoparticle raw materials market. One of the best methods for efficiently delivering antibiotics, nucleic acid therapies, and cytotoxic chemotherapeutic drugs is the use of liposome nanoparticles or LNPs. The main reason for their success is their intriguing physicochemical behaviors, which include high bioavailability, multiple routes of administration, large-scale production, intrinsic blood-brain barrier crossing ability, and the capacity to deliver macromolecules like DNA, oligonucleotides, and proteins. Oral and parenteral medication delivery methods can be based on solid lipid nanoparticles. The advantages of polymeric nanoparticle and lipid emulsion systems combined in SLNs also solve the problems with in vivo stability and timing that plague both polymeric nanoparticles and traditional drug delivery methods.
The growth of the lipid nanoparticle raw materials market is restricted due to the burst release system. The possibility of systemic burst release is a significant hurdle in LNPs research. Changes in the lipid matrix, surfactant content, and production-related factors (such as temperature) can all have a substantial impact on the drug-release profile of LNPs. Furthermore, critical elements of the manufacturing process, such as temperature, surfactant content, and intrinsic properties of the lipid matrix, influence the release of the medication from lipid nanoparticles.
The therapeutic potential of genetic therapeutics based on RNA or DNA is extraordinary since almost every illness may be addressed by editing faulty genes, producing a helpful protein, or suppressing a dangerous gene. To bring these macromolecules into the inside of target cells, nucleic acid polymer-based medicines need advanced delivery mechanisms. The ability of LNPs to prevent encapsulated nucleic acids from degrading is one of its main benefits. Around the genetic material, the cationic lipids form a barrier that prevents them from being broken down by nucleases or exposed to extreme weather. As said, the technology for small molecule medicinal compositions using LNPs is very advanced. Proteins and nucleic acids are examples of biological components that are encapsulated in LNP, a recently developed kind of medicinal formulation.
Which Product Segment Led the Lipid Nanoparticle Raw Materials Market?
By product, the kits segment led the market with the largest share in 2023. Different formulations of lipid nanoparticles (LNPs) may be needed for different therapeutic uses. With the use of kits, LNPs may be created with specific target cells, tissues, and therapeutic goals in mind. Kits make it easier to optimize iteratively, so you may gradually increase the stability, safety, and efficiency of LNP formulations.
For instance,
How the Infectious Diseases Segment Dominated the Lipid Nanoparticle Raw Materials Market?
By disease indication, the infectious diseases segment dominated the market in 2024. The development of novel and current medications in nano-sized carriers holds the potential to address a number of issues related to the management of these illnesses, such as low on-target bioavailability, drug accumulation that is subtherapeutic in microbial havens and reservoirs, and low patient adherence brought on by toxicities connected to the medications and lengthy treatment regimens. Nanocarriers may also be utilized to create vaccinations, which are a powerful tool in the battle against infectious illnesses.
As of 2023, infectious illnesses continue to be one of the major global causes of morbidity and mortality, accounting for almost 52 million (33%) of all deaths globally. There is still a 50% chance that infectious illnesses may resurface and spread among the world's population. According to current data, there are an estimated 14 million pediatric fatalities worldwide each year, with vaccine-preventable illnesses accounting for 70% of these deaths and poor nations accounting for 99% of all pediatric deaths.
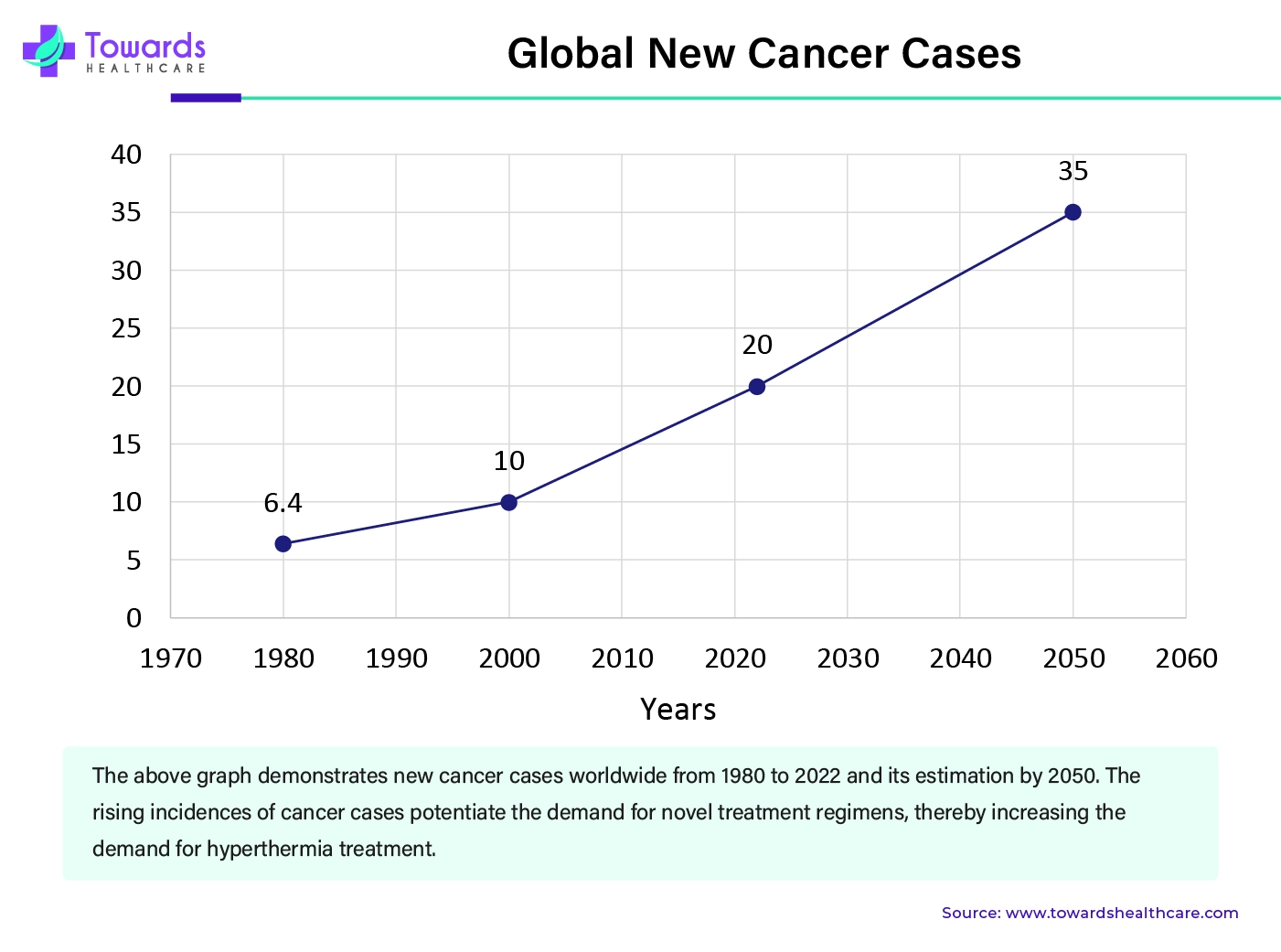
By disease indication, the cancer segment is expected to grow at the fastest rate during the forecast period. The most cutting-edge and extensively used delivery methods for nucleic acids and small compounds are lipid-based NPs. In the context of cancer immunotherapy, lipid-based NPs have the ability to ex-vivo construct cancer cell treatments with a comparable level of effectiveness to that of other non-viral or viral vectors, in addition to delivering small compounds and mRNA therapies in vivo to reach tremendous antitumor efficacy.
The rising worldwide cancer incidence, its uneven effect on vulnerable populations, and the urgent necessity of tackling cancer inequalities are highlighted by the IARC estimates. The greatest data sources that countries will have in 2022 will serve as their foundation. For 2022, it was projected that there would be 20 million new cases of cancer and 9.7 million deaths from the disease. Five years after receiving a cancer diagnosis, an estimated 53.5 million individuals were still living. Five percent of people will have cancer in their lives; nine percent of men and twelve percent of women will die from the disease.
Why Did the Therapeutics Segment Dominate the Lipid Nanoparticles Raw Materials Market?
By application, the therapeutics segment led the market with the largest share. Because of their chemical and physical instability, therapeutic proteins are extremely delicate molecules. In addition to keeping the therapeutic protein structure safe from degradation, the lipid nanoparticle shape makes the protein more bioavailable and capable of passing across membranes. Lipid nanoparticles meet all the necessary conditions to be utilized as an ideal drug delivery mechanism, including the ability to entrap proteins and other hydrophilic and lipophilic substances. Therapeutic proteins have significant limitations that can be addressed, and entirely new prospects can be opened by encasing the therapeutic proteins in lipid nanoparticles.
By application, the research segment is estimated to grow at the fastest CAGR during the forecast period. At the vanguard of the quickly evolving subject of nanotechnology are solid lipid nanoparticles, which have a variety of potential uses in clinical treatment, research, medication delivery, and other diverse disciplines. Lipid nanoparticles provide a promising avenue for the development of novel therapies because of their distinct size-dependent characteristics. The capacity to encapsulate medications into nanocarriers presents a novel drug delivery model that may be applied to secondary and tertiary drug targeting. Therefore, researchers have focused a lot of attention on solid lipid nanoparticles because they show tremendous potential for achieving the objective of regulated and site-specific drug delivery.
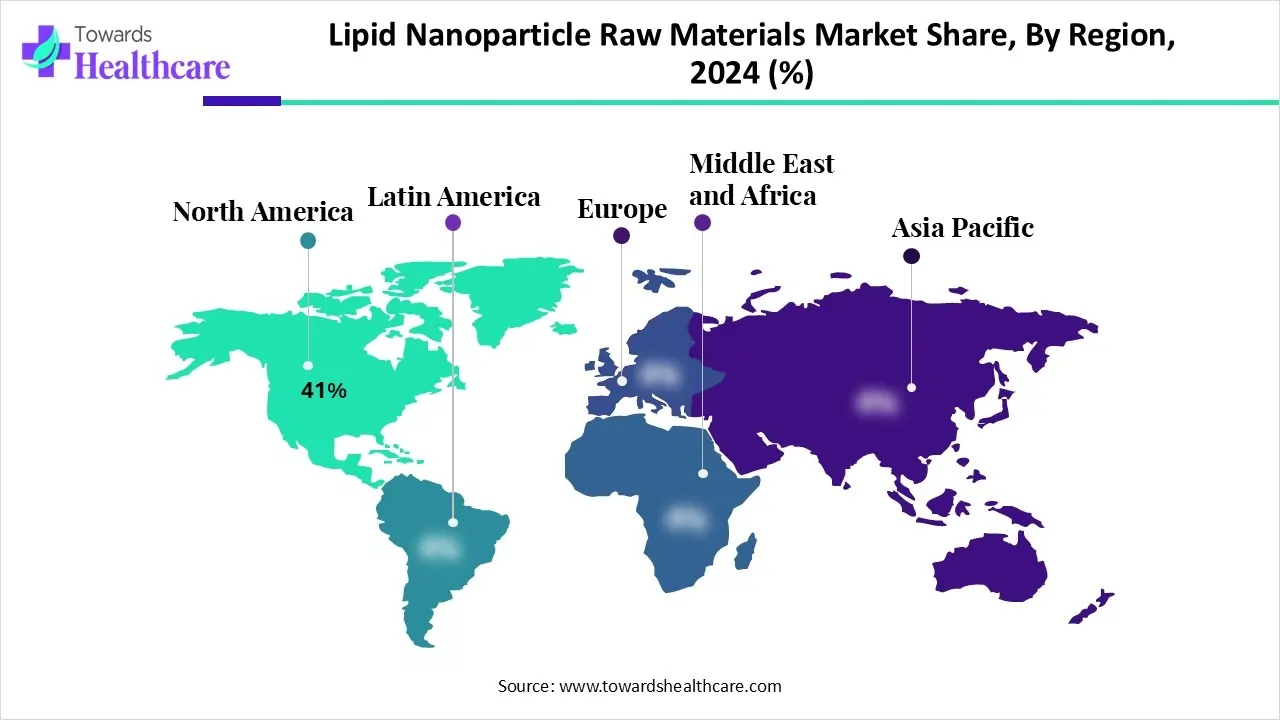
By region, North America dominated the market share by 41% in 2024. North America is known for its advancements in technology and healthcare. A huge amount of investment is made to conduct proper research and development in order to fulfill the healthcare demand in different categories. The U.S. is among the dominant countries that promote the growth of the lipid nanoparticle raw materials market globally and in the North American region. Several factors contribute to that dominance, among which one is research and development in the healthcare sector.
For instance,
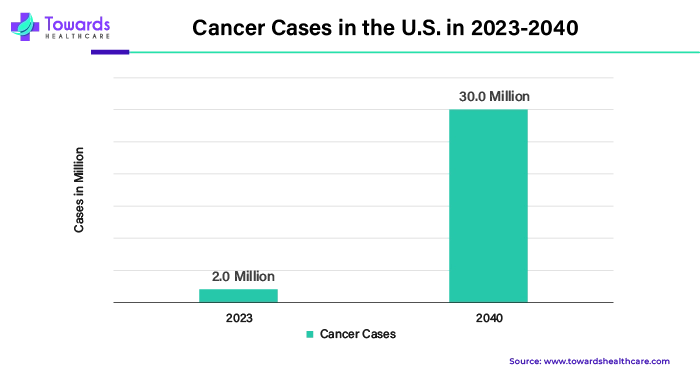
The growing prevalence of infectious diseases brought on by the application of lipid nanoparticles in infection therapy is another factor propelling the growth of the lipid nanoparticle raw materials market in the U.S.. Every year in the US, there are more than 48 million instances of diarrhea, which leads to 128,000 hospital admissions and 3,000 deaths from diarrheal illnesses. Lipid nanoparticle research is becoming increasingly significant due to the rising number of cancer patients in the United States. Based on U.S. demographic data, ten million cancer-related deaths and twenty million new instances of the illness are anticipated each year.
Canada is the second-largest contributor to the expansion of the lipid nanoparticle raw materials market, behind the U.S. 9 million dollars in funding will be used by 2024 to integrate researchers into health system organizations nationwide. In order to meaningfully contribute to strengthening Canada's healthcare system and guarantee that Canadians receive high-quality medical care, the government of Canada is funding researchers across the nation who are working with health system organizations. Researchers may assist in identifying and implementing answers to the problems that this nation's health systems are confronting by becoming embedded in organizations that support the health system.
For instance,
Asia-Pacific is projected to be the fastest-growing region during the forecast period. The growing research and development activities, owing to increasing investments and collaborations, augment market growth. The availability of suitable manufacturing infrastructure encourages manufacturers to set up manufacturing facilities for producing LNPs. The presence of key players fosters market growth. Key players like WuXi Biologics, Shin-Etsu Chemical, and VAV Life Sciences hold a major share of the market in Asia-Pacific. Countries like China and India majorly contribute to exporting raw materials for the preparation of lipid nanoparticles.
China is a global exporter of pharmaceutical raw materials. Beckman Coulter and JenKem Technology are major biotech companies that have set up their facilities in China. The Chinese government actively supports the development of innovative drug delivery systems through initiatives and funding. The clinical trial infrastructure is rapidly expanding in China, allowing researchers to test their novel drugs in humans.
Europe is expected to grow at a notable rate in the foreseeable future. Favorable government support and suitable regulatory frameworks propel the market. The growing research for novel drug delivery systems and increased mRNA vaccine production also leads to market growth. The European Pharmacopoeia Commission (EPC) adopted three new general texts on November 2024, relating to the production and quality control of mRNA vaccines and their components. Companies like Moderna are securing tenders for supplying their mRNA COVID-19 vaccines in the European Union.
The UK government has established a Sector Plan to boost the life science ecosystem, grow the region’s economy, and improve people’s lives. It funded £2 billion for the plan, along with the funding from UK Research and Innovation (UKRI) and the National Institute for Health and Care (NIHR). It wants NHS patients across the UK to be able to benefit from timely access to safe, clinically effective, and cost-effective new medicines.

| Company Name | Hopewell Therapeutics |
| Headquarters | Massachusetts |
| Recent Development | In May 2024, a biotechnology company, Hopewell Therapeutics, is dedicated to the discovery, synthesis, and development of the next generation of tissue-targeted lipid nanoparticles (ttLNPs) for the delivery of genomic medicines to patients. Today, the company announced that it will virtually attend the American Society of Gene and Cell Therapy (ASGCT) 27th Annual Meeting this week in Baltimore, MD, to present preclinical data showing the progression of its vaccine, oncology, and pulmonary programs. |
| Company Name | Cytiva |
| Headquarters | U.S. |
| Recent Development | In July 2023, the commercial formulation method NanoAssemblr was introduced by Cytiva, a leading company in the life sciences industry, with the aim of producing lipid nanoparticle therapeutics for both clinical and commercial use. Companies can use the fully compliant and automated instrumentation of the NanoAssemblr commercial formulation system to bring these medications to market by utilizing a single, scalable mixing technology for all phases of development, from large-scale manufacturing to product development and research. |
By Product
By Disease Indication
By Application
By Region
January 2026
December 2025
November 2025
October 2025