November 2025

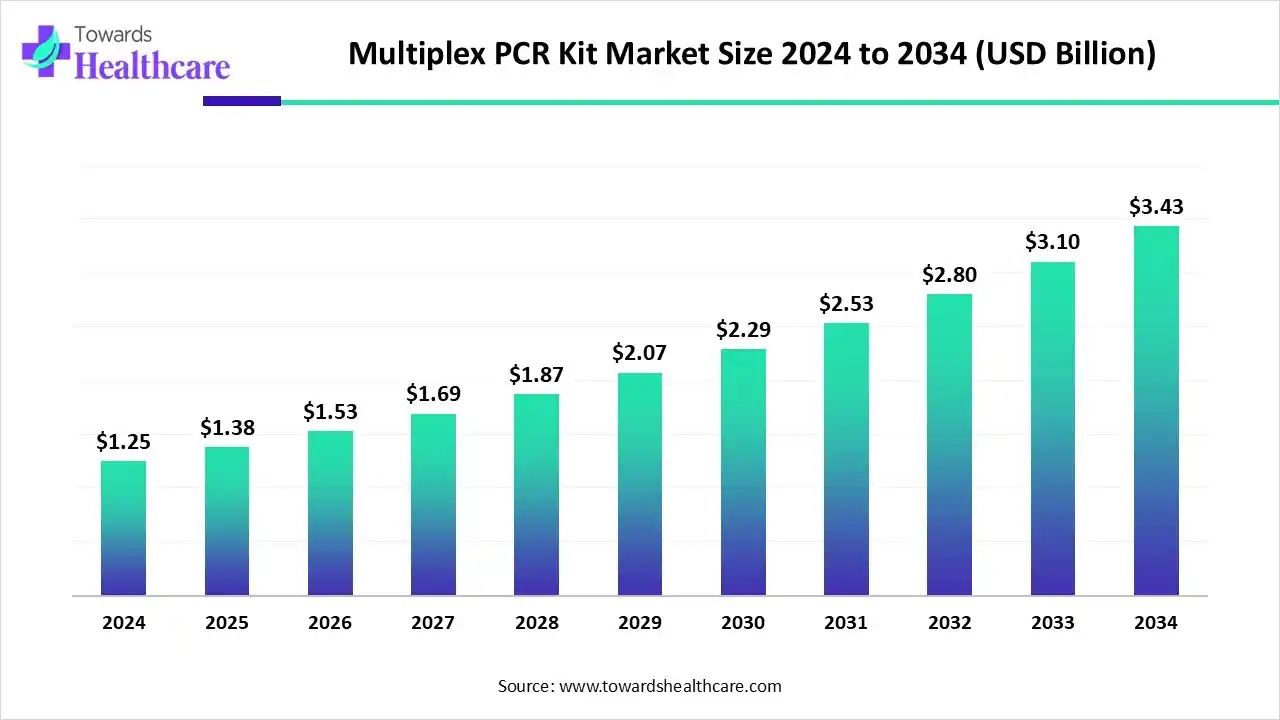
The global multiplex PCR kit market size is estimated at US$ 1.25 billion in 2024 and is projected to grow to US$ 1.38 billion in 2025, reaching around US$ 3.43 billion by 2034. The market is projected to expand at a CAGR of 10.64% between 2025 and 2034.
The multiplex PCR kit Market is growing because of the rising demand for advanced diagnostic solutions, specifically for genetic disease research, oncology, and infectious disease, as well as the increasing burden of chronic diseases. North America is dominant in the market due to government support in the adoption of novel healthcare devices and technology, while the Asia Pacific is the fastest growing a massive patient base and expanding research and development activities.
| Table | Scope |
| Market Size in 2025 | USD 1.38 Billion |
| Projected Market Size in 2034 | USD 3.43 Billion |
| CAGR (2025-2034) | 10.64% |
| Leading Region | North America by 35% |
| Market Segmentation | By Technology Type, By Product Type, By Application, By Region |
| Top Key Players | Thermo Fisher Scientific Inc., Bio-Rad Laboratories Inc., Agilent Technologies Inc., Roche Molecular Systems Inc., Takara Bio Inc., Promega Corporation, Illumina Inc., Meridian Bioscience Inc., Seegene Inc., GenScript Biotech Corp., Danaher Corporation (Beckman Colter / Cepheid), PerkinElmer Inc., Norgen Biotek Corp., Bioline (Meridian Life Science), New England Biolabs (NEB), Enzo Life Sciences Inc., Abcam plc, Eurofins Genomics, GeneFirst Ltd. |
The multiplex PCR kit market involves reagent and consumable kits enabling simultaneous amplification of multiple DNA or RNA targets in a single PCR reaction. These kits integrate optimized primer sets, polymerases, buffers, and master mixes compatible with conventional, quantitative, and digital PCR systems. They are critical in infectious-disease diagnostics, oncology genotyping, pharmacogenomics, forensic identification, and food-safety or environmental testing.
Multiplex PCR kits reduce assay time, conserve samples, and enhance diagnostic throughput, supporting point-of-care as well as high-throughput laboratory workflows. The market includes kits for research and clinical use, covering nucleic-acid extraction compatibility, assay validation, and integration with automated thermocyclers and detection platforms across life-science and clinical laboratories worldwide.
This AI-driven technology enhances DNA profiling, providing a rapid and more reliable outcome for medical, security, and forensic applications. The AI-driven kit optimizes the performance of PCR, improving amplification success for degraded or trace DNA samples, significant for healthcare diagnostics. Machine learning enhances PCR efficiency, allowing fast results while lowering errors in various applications, from medical care to environmental monitoring. AI-driven technology offers a quick, precise, and effective process for PCR reagent design, significantly improving global diagnostic preparedness by improving primers and probes for the timely detection of infectious health conditions.
By technology type, the real-time (qPCR) multiplex PCR segment led the multiplex PCR kit market with approximately 45% share, as real-time PCR reactions are conducted in a closed system, the challenges of contamination have been considerably reduced. Real-time PCR protocols are generally built around singleplex experiments, so switching to multiplexing requires adjustments to the protocol. Real-time PCR has become one of the broadly used techniques of gene quantitation as it has a large dynamic range, boasts marvelous sensitivity. RT reaction optimized to increase cDNA yield, for the subsequent Q-PCR amplification.
On the other hand, the digital multiplex PCR segment is projected to experience the fastest CAGR in the multiplex PCR kit market from 2025 to 2034, as the multiplexing process instantaneously identifies and quantifies closely related deoxyribonucleic acid sequences in complex mixtures. The dPCR concept is continuously enhanced by the progress of microfluidics and micro and nanofabrication, and various complicated technologies. Digital PCR allows absolute quantification and identification of minority sequences against comparable majority sequences, such as somatic mutations.
By product type, the multiplex PCR reagent kits segment led the multiplex PCR kit market in 2024 with approximately 40% share, as multiplex PCR is a broadly used technique for the amplification of multiple targets in a single PCR technology. Multiplexing saves precious time, reagents, and samples, and allows simultaneous comparison of multiple amplicons. A multiplex PCR reagent kit contains the required machinery for a Polymerase Chain Reaction (PCR) that instantaneously amplifies multiple DNA targets in a single reaction.
On the other hand, the target-specific diagnostic panels segment is projected to experience the fastest CAGR from 2025 to 2034, as target-specific diagnostic PCR panels provide numerous benefits, specifically in the rapid and precise recognition of pathogens and genetic variations, which eventually lead to better-informed medical and research decisions. The rapid turnaround time allows physicians to make sooner, more confident treatment choices.
By application, the infectious disease diagnostics segment led the multiplex PCR kit market in 2024 with approximately 55% share, as multiplex PCR (mPCR) has evolved as a transformative diagnostic device, allowing the simultaneous amplification of multiple target DNA/RNA sequences from various pathogens in a single reaction. Molecular tests instantaneously detect a range of pathogens and the presence of various genes causing antibiotic resistance, lowering treatment with broad-spectrum antibiotics and turnaround time (TAT). This enables physicians to detect bacterial and fungal pathogens within hours, significantly reducing diagnostic timelines and facilitating early, targeted antimicrobial therapy.
On the other hand, the oncology and genetic mutation testing segment is projected to experience the fastest CAGR in the multiplex PCR kit market from 2025 to 2034, as multiplex PCR allows the immediate amplification and identification of numerous target sequences in a single reaction. It offers increased reliability by built-in internal controls and is important for applications such as cancer biomarker detection, rare disease screening, and antimicrobial resistance profiling.
Globally, new oncology cases are projected to increase significantly over the next few decades, driven by population growth and aging. Estimates suggest approximately 30.5 million cases by 2025, 26 million by 2030, and around 35 million by 2050, reflecting a 77% rise from 2022's estimated 20 million. While growth will be pronounced everywhere, low and medium-Human Development Index (HDI) countries will experience a proportionally higher burden.
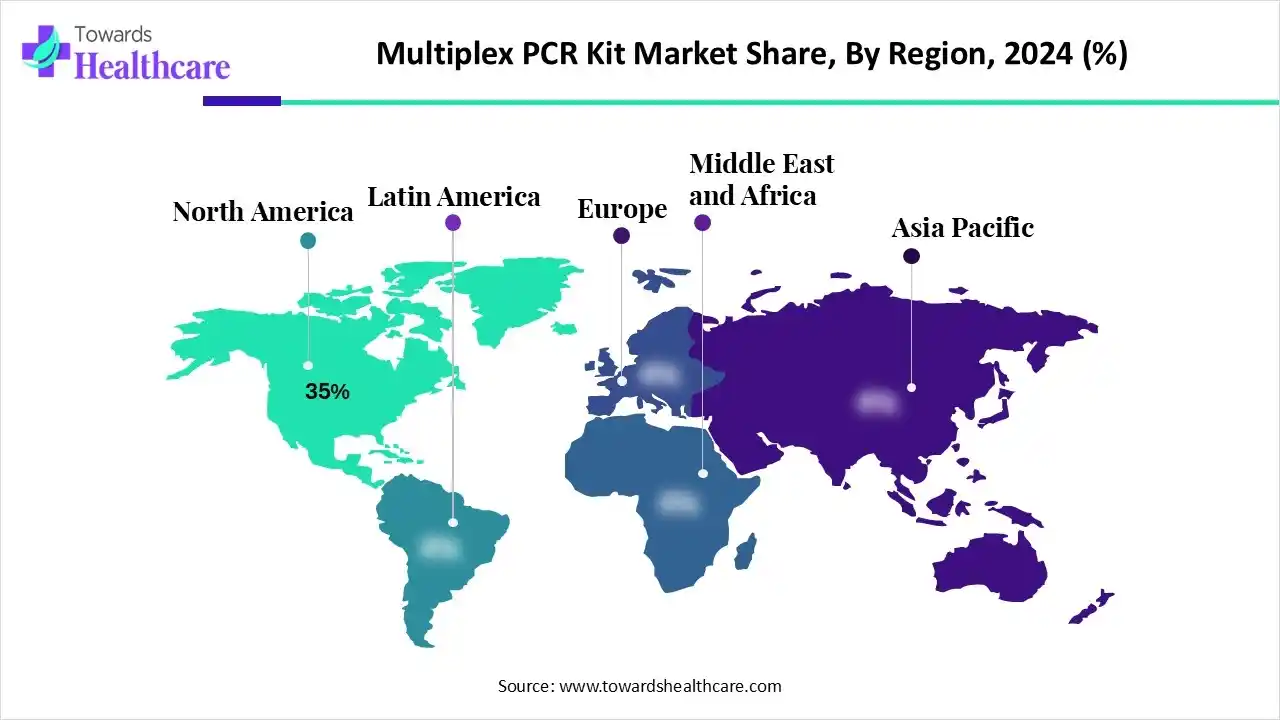
North America is dominant in the market in 2024, with approximately 35% share, due to the growing prevalence of chronic and infectious disorders, including a noteworthy cancer burden, which drives a strong demand for rapid and precise diagnostic instruments like multiplex PCR. The region is a hub for major leading companies in the multiplex PCR market, such as Thermo Fisher Scientific, Bio-Rad Laboratories, and QIAGEN, which spend heavily in innovation and novel product launches, which contribute to the growth of the market.
In the U.S., the growing academic and pharmaceutical sectors in this region use multiplex PCR for genetic studies, drug discovery, and biomarker validation. Hospital and diagnostic laboratories are major drivers of the market due to high patient volumes and the requirement for fast and precise disease identification. The U.S. has a sophisticated medical care system with modern diagnostic laboratories, which facilitates the adoption of novel and ground-breaking diagnostic technologies, which drives the growth of the market.
A favorable ecosystem, supported by organizations like the National Institutes of Health (NIH), fosters innovation and investment. The NIH committed $814 million to gene-editing projects as of September 2024. Projects funded in July 2023 included over $140 million for the Somatic Cell Genome Editing (SCGE) program and $22 million for CRISPR-based therapies for neurological illnesses.
Asia Pacific is the fastest-growing region in the market in the forecast period, due to growing spending in medical care infrastructure, increasing demand for advanced molecular diagnostics, and the increasing prevalence of chronic and infectious diseases. The rising requirement for effective cancer screening and the rise of targeted medicine are increasing the use of multiplex PCR in various diseases.
For Instance,
China is experiencing significant investment and growth in targeted medicine, particularly in oncology, which is a significant growth area for multiplex PCR. Recent developments are focusing on improving accuracy, speed, and efficiency. This market is driven by the required for timely and accessible diagnostics. There is an increasing demand for transportable, quick PCR kits for use outside traditional healthcare settings.
Europe is a notable growing region in the market with the increasing prevalence of infectious diseases, developments in molecular diagnostics, and rising research in genomics and genetics. PCR technology is broadly applied in healthcare diagnostics, specifically for identifying rare disorders, infectious diseases, and oncology.
In Germany, the increasing burden of long-term disease and infectious diseases increases the demand for PCR tests. For instance, Germany is accomplished of doing about 430,000 PCR tests per day. The presence of major research centers and institutions such as RKI, which holds specialized units on enormously pathogenic bacteria, biological toxins, and viruses, drives the growth of the market.
The research and development (R&D) technology for the multiplex PCR kit significantly includes target identification and primer design, master mix formulation, analytical validation, specificity testing, and manufacturing and final quality control
Key Players: Thermo Fisher Scientific and QIAGEN
Clinical trials of a multiplex PCR kit focus on validating its performance as an in vitro diagnostic (IVD) rather than the ideal many-phase drug trial model. It involves pre-submission to government authorities, investigational device exemption (IDE), clinical performance assessment, government submission and review, and post-market scrutiny.
Key Players: Bio-Rad Laboratories and Roche Diagnostics
Patient services provided by multiplex PCR kits are Rapid diagnosis of infectious diseases, guiding antibiotic and antifungal therapy, genetic disease testing and counseling, and providing personalized medicine
Key Players: Hologic and Abbott Laboratories
By Technology Type
By Product Type
By Application
By Region
November 2025
November 2025
December 2025
November 2025
