February 2026

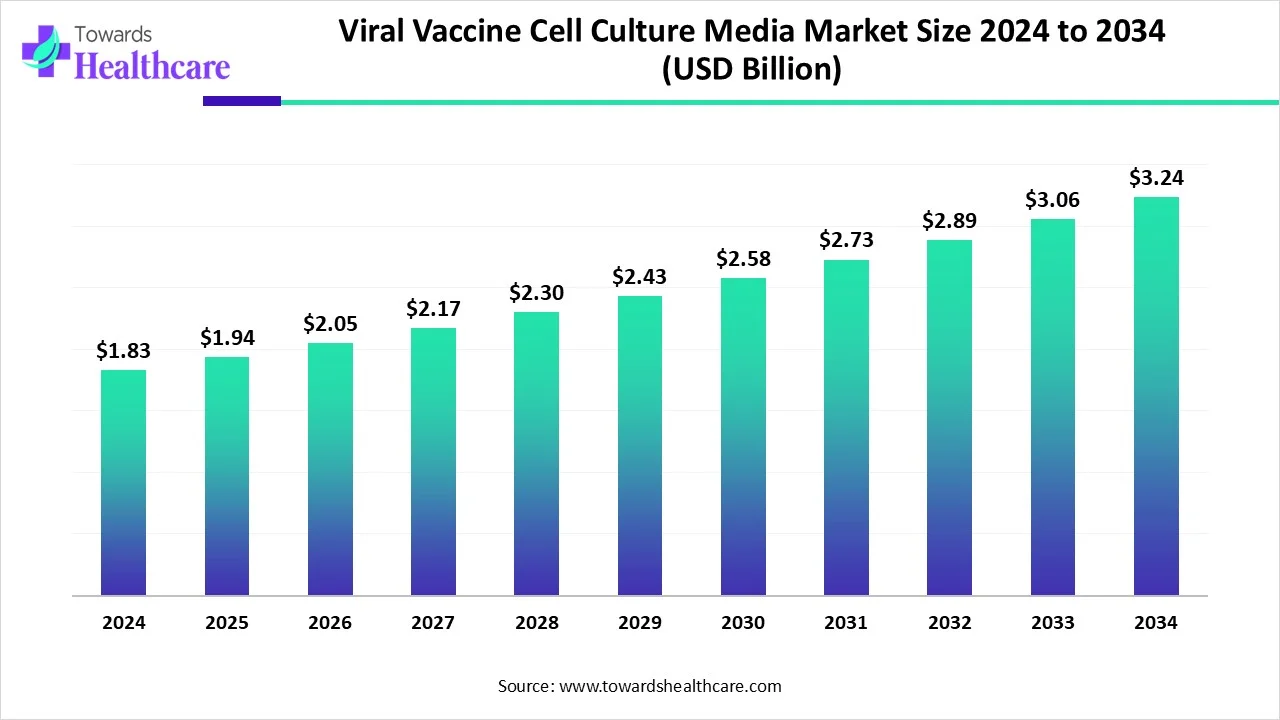
The global viral vaccine cell culture media market size is calculated at USD 1.83 in 2024, grew to USD 1.94 billion in 2025, and is projected to reach around USD 3.24 billion by 2034. The market is expanding at a CAGR of 5.84% between 2025 and 2034.
| Metric | Details |
| Market Size in 2025 | USD 1.94 Billion |
| Projected Market Size in 2034 | USD 3.24 billion |
| CAGR (2025 - 2034) | 5.84% |
| Leading Region | North America |
| Market Segmentation | By Cell Culture Type, By Cell Culture Media, By Scale of Operation, By Region |
| Top Key Players | Creative Biolabs, Jianshun Biosciences, Thermo Fisher Scientific, Merck, Sartorius, Xell, ATZ Labs, OPM Biosciences |
Vaccination is one of the most effective global health initiatives. Global vaccination initiatives significantly reduced the number of deaths attributable to infectious agents. The serious outbreaks caused by these novel viruses will be better controlled with the aid of vaccines. However, there are other obstacles to developing novel vaccines, including limited development periods and the ongoing evolution of virus strains. Inactivated viral vaccines are the most popular way to prevent disease. To meet the demands for vaccine production, there has been an increased focus on figuring out how to make the manufacturing process more efficient.
It has been shown that ML-based techniques have been effectively used for medium development. By concurrently fine-tuning several components, machine learning approaches might improve culture media and pinpoint the essential medium components for metabolic production and cell development. Since ML-assisted media optimization is based on data science and solely uses quantitative and statistical evaluation that is not influenced by personal experience, it will become a widely used approach.
Emerging Diseases & Rising Global Demand for Viral Vaccines
In recent years, the need for viral vaccinations has increased significantly due to the global growth in the occurrence of viral illnesses and pandemics. In particular, the COVID-19 pandemic has brought attention to how crucial immunization is to reducing the severity and spread of respiratory viral illnesses. Effective vaccine development necessitates efficient manufacturing using cell culture media, which act as a growth medium for viruses. Cell culture media are becoming increasingly necessary as vaccine producers seek to increase access and scale up supply in order to satisfy the enormous worldwide demand created by immunization campaigns.
High Cost of Products
In the approaching years, the market for cell culture medium is likely to be hindered by the high cost of cell biology research goods. To get accurate findings, high-quality media, reagents, and other goods related to the study on developing novel viral vaccines are required.
Are Initiatives and Programs Helpful for the Viral Vaccine Cell Culture Media Market?
The primary drivers of the worldwide viral vaccine cell culture media market are vaccination programs and government efforts aimed at preventing viral illnesses. To protect their populations from diseases that may be avoided by vaccination, several governments across the world have implemented national immunization programs. These programs provide a consistent demand for viral vaccines, which in turn drives up demand for the cell culture medium required for vaccine production. Another factor driving the market's growth is government funding for vaccine research and development.
By cell culture type, the suspension cell culture segment held the largest share of the viral vaccine cell culture media market and is estimated to grow at the fastest CAGR during 2025-2034. Compared to conventional adherent cell culture, suspension cell culture has a number of benefits that make it an effective tool for biomanufacturing and research. Modern biomanufacturing relies heavily on suspension cell culture, especially when it comes to the creation of vaccines, viral vectors, and biologics.
By cell culture media, the serum-free media segment captured the majority of the viral vaccine cell culture media market share in 2024. Many cell types are commonly cultured in serum-free conditions. Using serum-free medium for cell culture has various benefits, such as improved productivity and uniformity, as well as simpler downstream processing and purification. Additionally, growth factor combinations that are selective for particular cell types can be added to serum-free medium.
By cell culture media, the animal-free media segment is anticipated to witness the highest growth rate in the viral vaccine cell culture media market in the upcoming period. The need for morally sound and environmentally friendly solutions has increased dramatically in the rapidly changing field of scientific study. Because they are chemically defined, animal-free reagents provide higher uniformity and repeatability, which results in more trustworthy study findings. Lowers the possibility of animal pathogen contamination, guaranteeing cleaner and more trustworthy findings.
By scale of operation, the commercial segment dominated the viral vaccine cell culture media market in 2024 and is expected to be the fastest-growing during the predicted timeframe. Commercial culture media are offered as working solutions, liquid concentrates, or dehydrated or powdered forms. Logistics, operability, and process economics can all be impacted by media. Media-development services are provided by several media vendors and independent businesses.
North America dominated the viral vaccine cell culture media market in 2024. The biopharmaceutical sector in North America is highly developed and advanced, with the United States at the forefront. This region is home to several biotech and pharmaceutical businesses that manufacture vaccines, produce biologics, and conduct virology research.
In May 2025, for viruses that are prone to pandemics, HHS and NIH introduced a next-generation universal vaccination platform. The NIH built the BPL platform, which is entirely owned by the government. Public accountability, extreme openness, and the absence of economic conflicts of interest are all guaranteed by this strategy. Universal influenza vaccinations are expected to go through clinical testing in 2026, with FDA approval anticipated in 2029.
The federal, provincial, and territorial governments of Canada share responsibility for vaccination programs. The University of Manitoba (UM), in collaboration with the Universities of Alberta, Saskatchewan, and Calgary, for example, was awarded $57 million in vital funding in May 2025 to carry out world-class vaccine and biomanufacturing research and construct two new cutting-edge facilities. This investment puts the university in a strong position to play a major role in addressing future pandemic threats in Canada and globally.
Asia Pacific is estimated to host the fastest-growing viral vaccine cell culture media market during the forecast period. This increase is being driven by a greater understanding of the advantages of cell culture techniques. Major market players' conscious attempts to expand their presence in the Asia Pacific countries in an attempt to gain a significant share of the market also provide lucrative opportunities. Some of the main factors driving the growth of this regional segment include the presence of major players, the increasing regulatory approvals for cell-based vaccines, the increased focus on pharmaceutical and biopharmaceutical research, the continuous capacity expansion by biopharmaceutical manufacturers, and the rising funding for cell-based research.
Inactivated vaccine production has a lengthy history in China and is one of the most important types of vaccines produced there. Research on novel inactivated vaccines, aggressive promotion of large-scale inactivated vaccine manufacturing, and ongoing advancements in production technology and quality control are all priorities for China.
Examples of India's leadership in global health include the development of the first DNA vaccine against COVID-19 and the efforts underway to develop the first HPV vaccine for adolescent girls, which would prevent cervical cancer. Furthermore, by producing 65% of the world's vaccines, India has significantly improved health outcomes, especially for low- and middle-income countries. The thriving ecosystem of over 6,000 bio-startups has contributed to the thirteen-fold growth of India's bioeconomy in only ten years. To sustain this momentum, the industry must continue to support R&D, assist new entrepreneurs, and foster a robust startup ecosystem.
Europe is expected to grow significantly in the viral vaccine cell culture media market during the forecast period due to the increased government spending on avoiding these infectious illnesses and the escalating R&D efforts for these vaccines. The market's expansion in this area is anticipated to be driven by the widespread use of viral vaccines and the increasing number of initiatives to implement immunization strategies for viral infections.
The Department of Health and Social Care (DHSC) receives advice on research and development spending from the UK Vaccine Network, which brings together business, academia, and other funding agencies. The UKVN Project carries out the network's suggestions. Vaccines and vaccination technologies for infectious diseases that might spread to low- and middle-income countries (LMICs) are being developed as part of the initiative. The network also acts as a platform for collective learning and aids in the creation of policy tools.

In May 2024, according to Martinez Ferrada, the initiatives we're funding will improve our capacity to provide medications, immunizations, and treatments for Canadians. Innovation will be promoted nationwide by these partnerships among research hospitals, postsecondary educational institutions, and research centers. Canada will be prepared to address future health issues because of the efforts of seasoned scientists working at institutions at the forefront of innovation. (Source - University of Alberta)
By Cell Culture Type
By Cell Culture Media
By Scale of Operation
By Region
February 2026
January 2026
January 2026
January 2026