February 2026

The global drug desensitization market size was estimated at USD 670.03 million in 2025 and is predicted to increase from USD 724.57 million in 2026 to approximately USD 1465.41 million by 2035, expanding at a CAGR of 8.14% from 2026 to 2035.
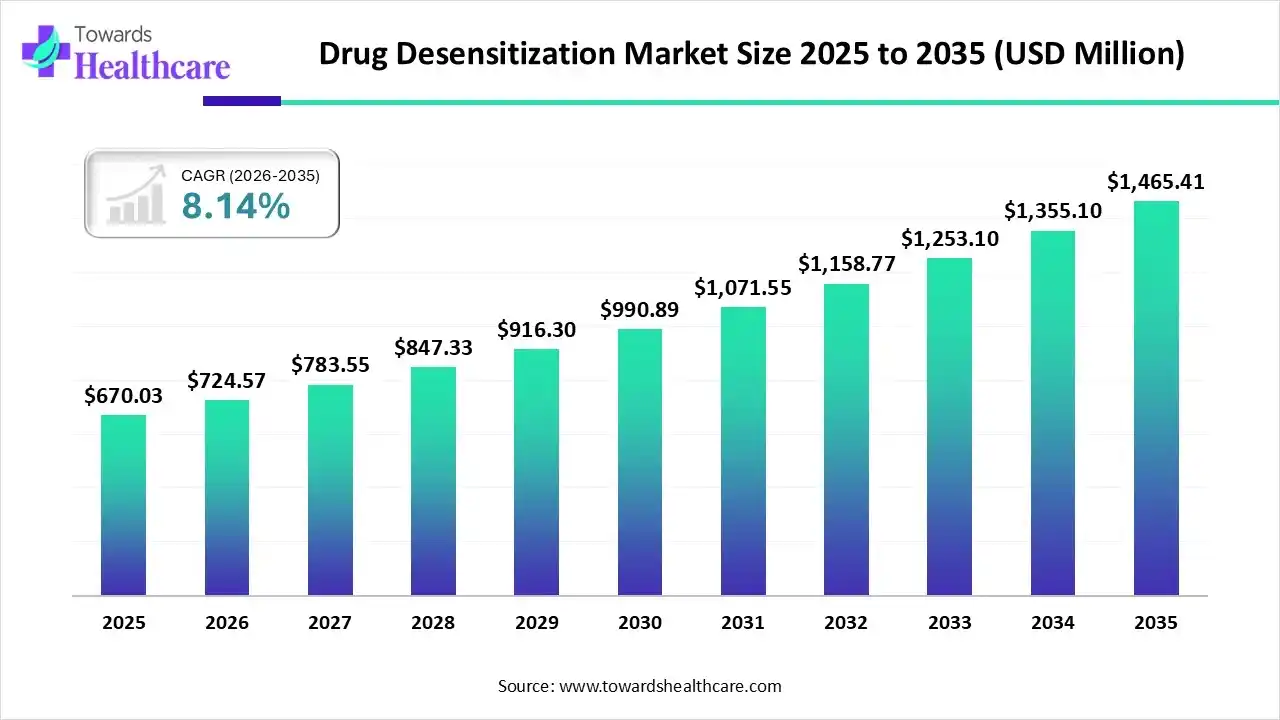
The drug desensitization market is growing as increasing its applications to manage both IgE-mediated and non-IgE-mediated drug hypersensitivity reactions.
The drug desensitization market is expanding due to its increasing clinical use in treating common infectious, metabolic, cardiovascular, and oncological diseases. It is frequently indicated for allergies to antibiotics (like penicillin or cephalosporins), chemotherapy agents, monoclonal antibodies (biologics), and NSAIDs. Drug desensitization enables allergic patients to safely receive essential medications they might otherwise avoid, allowing critical treatments for serious conditions like infections, cancer, autoimmune disorders, and chronic inflammation while reducing allergic reaction risks. This approach can be life-saving for patients dependent on specific drugs.
Integration of AI-driven technology in drug desensitization drives the growth of the market, as the AI-driven tools process massive amounts of patient information, including skin prick tests, specific IgE levels, and healthcare histories, to identify hidden correlations and predict allergic responses more reliably than traditional diagnostic processes. AI-based technology drives real-time analysis of multi-omics information to uncover novel biomarkers for allergy classification and prognosis. AI-based conjunctival provocation tests could offer more reliable, quantitative biomarkers for both diagnosis and therapy in allergic conjunctivitis. AI-based technology is an advantage and a safe therapeutic tool for selected high-risk desensitization events, mitigating the risks.
Rapid drug desensitization (RDD) allows first-line therapies in patients with instant drug hypersensitivity reactions (DHR) to chemotherapeutic drugs (ChD) and monoclonal antibodies (mAb).
Immunotherapy treatment is based on a century-old concept that the immune system responds to precise allergens that trigger allergy symptoms.
Personalized medicine lowers side effects, improves drug efficacy, and optimizes treatment results, which shows the essence of personalized medicine in the pharmaceutical field.
| Key Elements | Scope |
| Market Size in 2026 | USD 724.57 Million |
| Projected Market Size in 2035 | USD 1465.41 Million |
| CAGR (2026 - 2035) | 8.14% |
| Leading Region | North America by 39.5% |
| Market Segmentation | By Therapy Type, By Drug Class, By End-User, By Sales Channel, By Region |
| Top Key Players | ALK-Abelló A/S, Stallergenes Greer, Allergy Therapeutics plc, HAL Allergy Group, Merck KGaA, HollisterStier Allergy |
Which Therapy Type Led the Drug Desensitization Market in 2025?
In 2025, the sublingual immunotherapy (SLIT) segment held the dominant market share with approximately 66.1% share in 2025, as sublingual allergen immunotherapy (SLIT) is an aetiological management for allergic disorders such as allergic rhinitis, rhinoconjunctivitis, and asthma. It is available for the treatment of rhinitis, rhinoconjunctivitis, and allergic asthma, when the allergic component plays an important role in the clinical expression of the disease in both adults and children, with no legal age restriction.
SLIT Tablets
Whereas the SLIT Tablets segment is the fastest-growing with a CAGR of approximately 9.5% in the market, as SLIT is comparatively safe and efficient for the treatment of rhinitis and asthma caused by allergies to dust mites, cat dander, and tree pollens. SLIT meaningfully lowers symptoms of allergic rhinitis and asthma, leading to decreased use. SLIT is safe for both children and adults. SLIT-tablets provide specific advantages over injection immunotherapy.
Why did the Antibiotics Segment Dominate the Market in 2025?
The antibiotics segment is dominant and fastest-growing in tissue regenerative therapy, with approximately 42.2% share in 2025, as antibiotics desensitization shows a last-line choice for patients with no substitute therapies, where the advantages of this intensive process outweigh the potential harm from medicine exposure. Antimicrobial desensitization technology aims to establish a temporary state of tolerance to drugs that may otherwise cause hypersensitivity reactions.
Monoclonal Antibodies
Whereas the monoclonal antibodies segment is the fastest-growing in the market, as the important advantages of monoclonal antibody treatments are that they’re made to attach to a specific target. This means lower adverse effects than other treatments. Monoclonal antibodies use this tool to target damaging cells or substances in the body. Healthcare providers use monoclonal antibodies to treat significant types of illnesses and situations.
Why did the Hospitals and Specialty Clinics Segment Dominate the Market in 2025?
The hospitals and specialty clinics segment is dominant in the drug desensitization market with approximately 45.8% share in 2025, as drug desensitization permits the reintroduction of culprit drugs to extremely sensitized patients in need of first-line therapies. Drug desensitization has developed as a therapeutic modality that enables patients with drug allergies to receive the sensitizing medicine safely. Drug desensitization is the provisional induction of tolerance to a sensitized medicine by administering slow increments of the drug, starting from a small amount to a full beneficial dose.
Homecare/Self-Administration
Whereas the homecare/self-administration segment is the fastest-growing with a CAGR of approximately 10.4% in the market, as it allows patients to safely receive essential, first-line therapies like chemotherapy, antibiotics, or aspirin despite previous severe allergic reactions. It enables continued treatment for chronic conditions such as cardiovascular or musculoskeletal disease in patients with aspirin-exacerbated respiratory challenges.
Why did the Offline Segment Dominate the Market in 2025?
The offline segment is dominant in the drug desensitization market, with approximately 75.8% share in 2025, as offline channels remain dominant for emergency or specialized medicine requirements, where instant physical availability is critical. Consumers also perceive local, physical pharmacies as supplementary, trustworthy, and reliable, associated with online platforms, predominantly concerning the authenticity of the medicines. Offline pharmacies have flawlessly integrated technology with medical care, providing residents with the convenience of procuring medicines and wellness.
Online Pharmacies
Whereas the online pharmacies segment is the fastest-growing with a CAGR of approximately 10.9% in the market, as this pharmacy is a simple and convenient way to buy drugs. It is predominantly advantageous for (a) people who live far away from medical stores. Online pharmacies also offer competitive prices due to lower overhead expenses compared to physical pharmacies. It is convenient to order medicines from online drug stores confidentially, as well as to get free delivery.
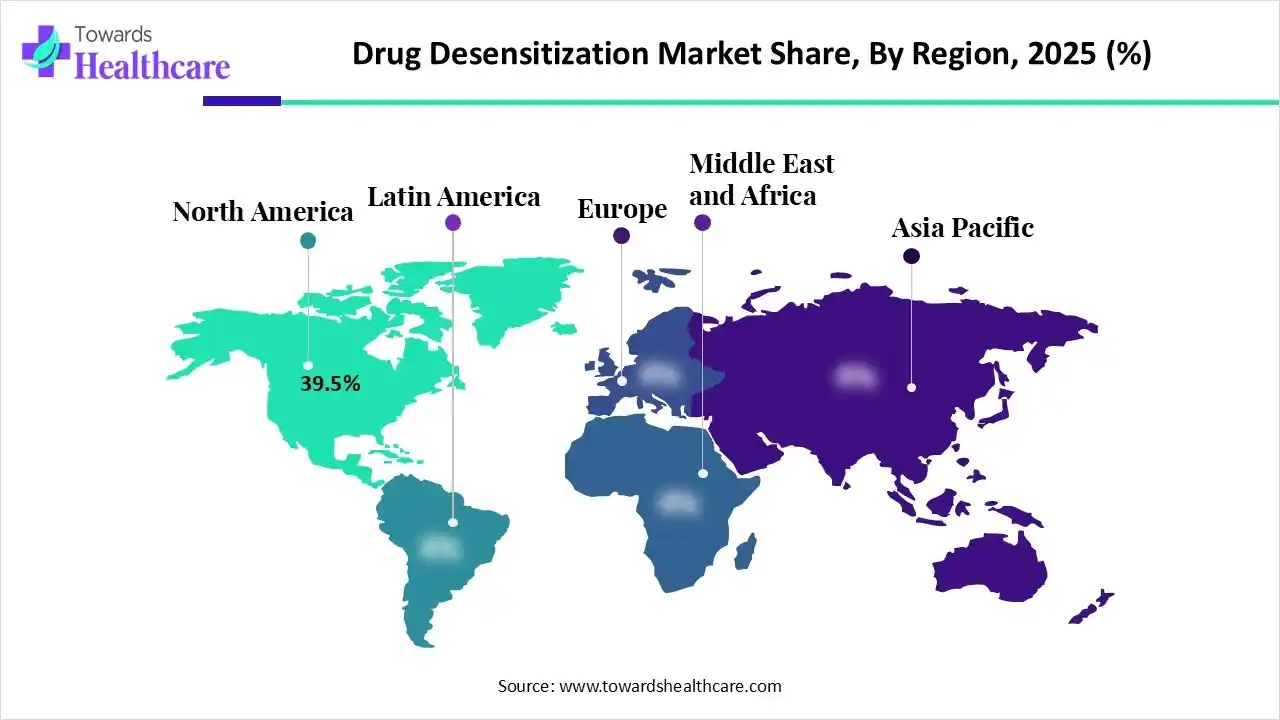
In 2025, North America dominated the drug desensitization market with approximately 39.5% share, as Well-established medical centers and high investment in clinical research, specifically for rapid drug desensitization (RDD), enhance safety and efficacy. In developed countries and urban areas, an increasing trend in allergy prevalence can be observed, likely attributed to the impact of environmental pollution. Strong RandD investments, rapid growth in biotechnology and precision medicine, and rising demand for innovative therapies.
For Instance,
U.S. Market Trends
In the U.S. strongly investment in research and development (RandD). This region provides an advanced financial infrastructure, involving venture capital organizations, angel investors, and sophisticated capital. The regulatory system plays a significant role in driving innovation and economic growth, providing significant financing to early-stage firms with new healthcare business ideas.
Asia Pacific is expected to see rapid growth in the drug desensitization market, driven by drug hypersensitivity reactions (DHRs) are recently become the third cause of allergy after rhinitis and asthma, with a noteworthy increase in prevalence in the Asia Pacific. Emerging countries possess major unique challenging factors for birth defects, which drive the growth of the market.
India Market Trends
In India, rising demand for medicines and increasing access to essential medicines. Innovations in significant healthcare technology supports to solve many population challenges. India's medical care is digitally transmuting to create combined, patient-centric solutions, improved healthcare delivery, and operational effectiveness, which increases the need for drug desensitization services.
Europe is significantly growing in the drug desensitization market, due to rising regulatory support, such as allergy treatment programs, which involve DPT and one-bag RDD-protocols, enabling patients to continue critical antineoplastic treatments despite IDHRs. A comprehensive practical guide on the methodological aspects of implementing acute-onset intravenous hypersensitivity delabeling and RDD for a broad range of drugs, which drives the growth of the market.

| Company | Headquarters | Latest Update |
| ALK-Abelló A/S | Denmark | In June 2025, ALK presented comprehensive data on two novel paediatric AIT tablets and a nasal adrenaline spray. |
| Stallergenes Greer | Switzerland | Stallergenes Greer advances allergy treatment via clinical trials and real-world studies and produces high-quality data. |
| Allergy Therapeutics plc | United Kingdom | Allergy Therapeutics plc made significant strides in the progress of its allergy desensitization portfolio. |
| HAL Allergy Group | United States | HAL Allergy is included in an ambitious clinical trial program to advance medicines with blockbuster strength for respiratory allergies. |
| Merck KGaA | Germany | Merck KGaA is seriously focused on increasing its oncology and immunology portfolio, with noteworthy activity in developing antibody-drug conjugates and advancing targeted therapies for immune-driven diseases. |
| HollisterStier Allergy | United States | Jubilant HollisterStier Allergy is pleased to announce that it has received zero observations following a recent inspection conducted by the United States Food and Drug Administration, Center for Biologics Evaluation and Research (CBER). |
By Therapy Type
By Drug Class
By End-User
By Sales Channel
By Region
February 2026
February 2026
February 2026
February 2026