February 2026

The global monoclonal antibodies market size is calculated at US$ 254.89 in 2024 billion, grew to US$ 286.6 billion in 2025, and is projected to reach around US$ 823.31 billion by 2034. The market is expanding at a CAGR of 12.44% between 2025 and 2034.

The use of monoclonal antibodies is increasing due to growing demand for biologics for the treatment of various chronic diseases. This is increasing the development, clinical trials, and launches, leading to new collaborations among the companies. Moreover, the funding rounds are also being announced to accelerate their development. The use of AI is also increasing the development of new platforms for drug discovery and development by minimizing errors. Hence, the demand for mAbs in various regions is increasing. Thus, this is promoting the market growth.
| Table | Scope |
| Market Size in 2025 | USD 286.6 Billion |
| Projected Market Size in 2034 | USD 823.31 Billion |
| CAGR (2025 - 2034) | 12.44% |
| Leading Region | North America |
| Market Segmentation | By Product/Modality, By Therapeutic Area/Indication, By Development Stage/Offering, By Manufacturing/Service Model, By Production Technology, By Region |
| Top Key Players | Roche / Genentech, AbbVie, Johnson & Johnson (Janssen), Merck & Co. (MSD), Bristol-Myers Squibb (BMS), Amgen, Pfizer, Novartis, Sanofi, Eli Lilly & Co., AstraZeneca, Regeneron Pharmaceuticals, GSK, Seagen (ADC leader), Samsung Biologics (CDMO), Samsung Bioepis (biosimilars), Wuxi AppTec / Wuxi Biologics (CDMO), Lonza (CDMO), Biogen, Daiichi Sankyo (ADC partnerships) |
The monoclonal antibodies (mAbs) market covers discovery, development, manufacturing, distribution, and clinical use of monoclonal antibody therapeutics and related products such as naked mAbs, ADCs, bispecifics, Fc-engineered antibodies, as well as biosimilars. mAbs are used across oncology, autoimmune/inflammatory diseases, infectious diseases, ophthalmology, and more. They are used for boosting the immune response or to provide target-specific action. Thus, the market includes R&D services, CDMOs, clinical/commercial biologics manufacture, fill-finish, and downstream cold-chain distribution.
Growing mAb products: The industry is developing various new biologics as well as personalized medications with the use of mAbs. This, in turn, is supported by the funding provided by various sources. Moreover, new collaborations are also being formed to accelerate their development.
For instance,
The use of AI to identify the antibody structure as well as its binding affinities is increasing. It is also being used in the identification and discovery of disease targets. This helps in developing an antibody therapeutic with desired properties. At the same time, the pharmacodynamics and pharmacokinetics of the monoclonal antibodies can also be predicted using AI. Hence, its use in the development of monoclonal antibodies is increasing to enhance its quality and minimize the chances of errors or failures. This, in turn, accelerates the development process.
Advancing Genetic Engineering
The growing advancements in genetic engineering are driving the demand for monoclonal antibodies. With its use, the antibody sequencing can be tailored, which in turn improves the therapeutic action, binding affinity, and safety of the mAbs. It is being used for the development of various antibody-drug-conjugates, biosimilars, and bispecific antibodies, which enhance the applications of mAbs. The stability and yield of the cell lines used for the development of mAbs are also enhanced with it. Thus, this drives the monoclonal antibodies market growth.
High Price
For the development of the mAbs, specialized facilities and equipment are required, which are costly. This increases their development cost. Similarly, the cost associated with the mAb products is also high, which makes it difficult for the patient to afford them. Moreover, the delay in the regulatory approval can also add to this cost. Thus, this can limit their adoption rates.
Growing Bispecific Antibody Treatment Approaches
The use of mAbs for the treatment of various chronic diseases is increasing. This, in turn, is increasing the demand for bispecific antibodies due to their dual targeting action. Therefore, the development of new bispecific antibody therapies for targeting cancer cells is increasing. It also helps in reducing the chances of resistance and the need for combination therapies. They are also being used for the treatment of infectious diseases, as they can help in neutralizing the viral strains. Thus, this is promoting the monoclonal antibodies market growth.
For instance,
By product/modality type, the naked/classic monoclonal antibodies segment held the dominating share of the monoclonal antibodies market in 2024. They showed promising safety and efficacy with increased therapeutic use. Moreover, they were used for the treatment of various diseases. This contributed to the market growth.
By product/modality type, the antibody-drug conjugates (ADCs) segment is expected to show the highest growth at a notable CAGR during the upcoming years. Due to their targeted action, their pipeline is growing. This is increasing their approval rates. Furthermore, the growing R&D investments are promoting their development.
By therapeutic area/indication type, the oncology segment led the monoclonal antibodies market in 2024 and is expected to show the highest growth during the upcoming years. To deal with the increasing cases of cancer, mAbs were used due to their target-specific action. Moreover, they were also used in combination with other therapies. There was also a rise in the use of ADC and bispecific antibodies.
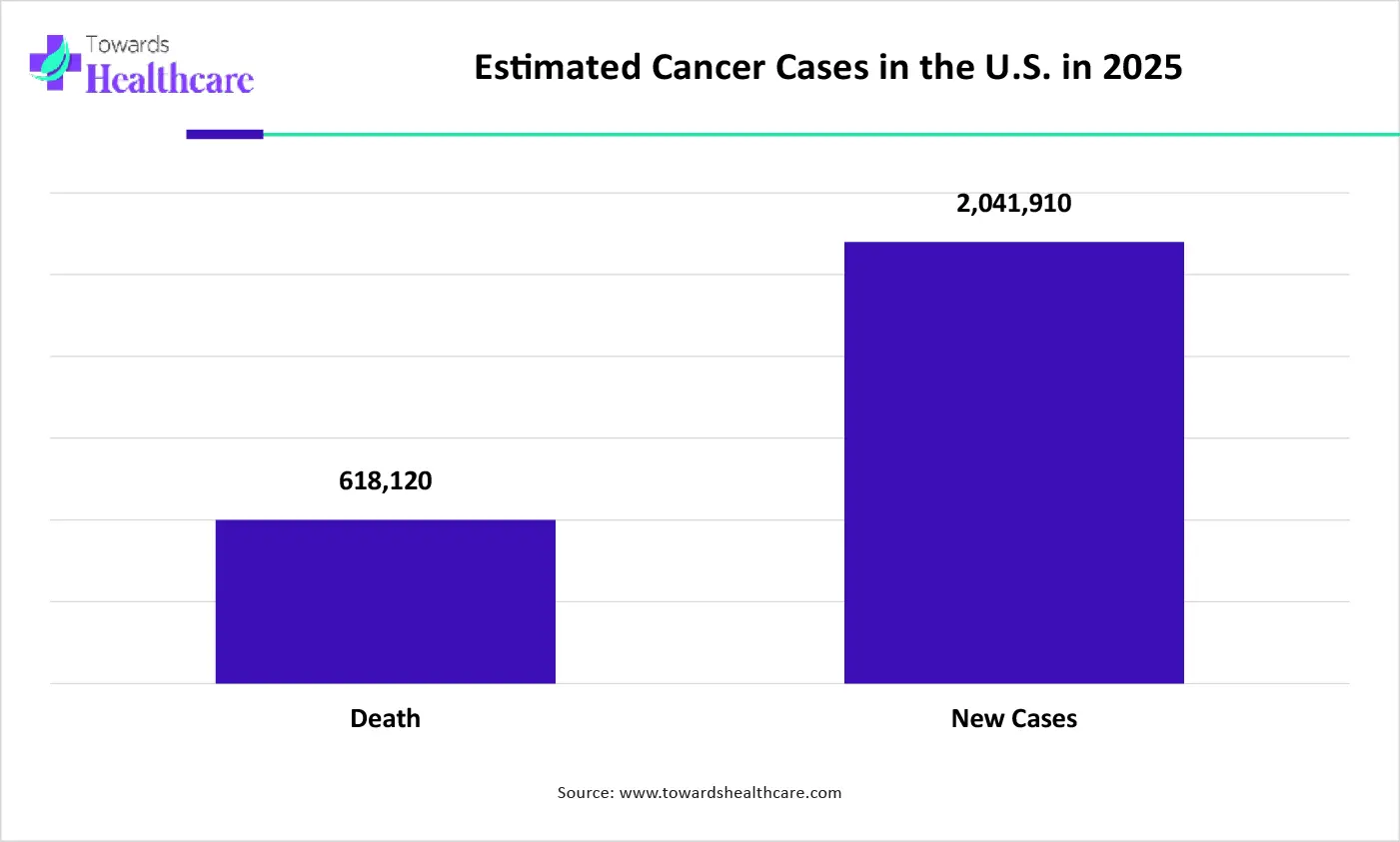
The graph represents the estimated number of cancer cases in the U.S. in the year of 2025. It indicates that there will be a rise in the number of cancer patients. Hence, it increases the demand for monoclonal antibodies for the development of new cancer therapies for their effective management. Thus, this in turn will ultimately promote the market growth.
By development stage/offering type, the commercial marketed therapeutics segment held the largest share in the market in 2024. They were used for the treatment of various diseases. This, in turn, increased their acceptance rates, which they were also supported by the reimbursement policies. Thus, this enhanced the market growth.
By development stage/offering type, the preclinical & clinical-stage assets segment is expected to show the fastest growth rate during the forthcoming years. The growing mAbs pipeline and R&D investment are supporting the development of new mAbs products. Moreover, the use of advanced technologies is also accelerating their clinical trials.
By manufacturing/service model type, the in-house integrated biomanufacturing segment led the market in 2024. They enhanced the quality of the product developed. At the same time, it also enhanced the R&D production rates. Moreover, it also provided flexibility, which enhanced the customized development of the mAbs.
By manufacturing/service model type, the CDMO/CMO manufacturing segment is expected to show the highest growth during the predicted time. Their use is increasing due to outsourcing trends. Moreover, they are also being used for the development of ADCs or biosimilars. Additionally, growing startups are also collaborating with them.
By production tech type, the mammalian cell expression systems segment held the largest share in the global market in 2024. They provided antibodies with improved safety and efficacy. Thus, they were widely used in the production of bispecific antibodies and ADCs. Hence, this promoted the market growth.
By production tech type, the single-use/intensified/continuous bioprocessing platforms segment is expected to show the fastest growth rate during the predicted time. They helped in reducing the cost and provided faster development of mAbs, with high yield. It also minimized the risk of contamination. This, in turn, is increasing their adoption rates
North America dominated the monoclonal antibodies market in 2024. Well-known biopharmaceutical industries are present in North America. They contributed to the development and commercialization of various monoclonal antibody products. Thus, this enhanced the market growth.
The growing research and development in the industries, as well as institutes in the U.S., is enhancing the development of various novel monoclonal antibody products. These developments are being supported by the government's investments and funding. Moreover, new clinical trials are also being conducted for mAb therapies.
The industries in Canada are launching new mAb products for the treatment of diseases such as cancer or autoimmune diseases. At the same time, advanced technologies are being used to develop new platforms and accelerate their development. Furthermore, the growing adoption of mAb therapies and biologics is increasing their demand.
Asia Pacific is expected to host the fastest-growing monoclonal antibodies market during the forecast period. The growing demand for biologics is increasing their manufacturing. This, in turn, is increasing the use of monoclonal antibodies. Thus, this contributes to the market growth.
The growing use of biosimilars and biologics in China is increasing their production, which in turn is increasing the demand for mAbs. At the same time, growing adoption of advanced technologies is increasing their manufacturing capacity. This is also being supported by the government.
Due to the growing disease burden, the use of mAb therapies and biologics is increasing in India. Moreover, the existing healthcare system is increasing its development and innovations. To make them widely available and affordable, support for the government and private sector is also being provided.
Europe is expected to grow significantly in the monoclonal antibodies market during the forecast period. The increasing research and development for the treatment of cancer and other diseases is increasing in Europe. This is increasing the demand for monoclonal antibodies for developing targeted therapies and biologics. Thus, this promotes the market growth.
The presence of biotech and pharmaceutical industries in Germany is increasing the use of mAbs for various purposes. At the same time, growing diseases are increasing the adoption of biosimilars. Thus, this is increasing the use of mAbs for their development, where the regulatory bodies are providing their support with faster approvals.
The growing research and development in the UK focusing on biologics is increasing the demand for mAbs. Moreover, new targeted therapies are also being developed with the use of bispecific antibodies. These developments are increasing the number of investments and funding, which are being used in their development and to enhance their accessibility.

In July 2025, after announcing the licensing agreement between AbbVie and IGI Therapeutics SA, the executive vice president, research and development, and chief scientific officer, at AbbVie, M.D. Roopal Thakkar stated that a new frontier in immuno-oncology that provides more durable and deeper responses by targeting multiple targets simultaneously, is being represented by multispecifics, including trispecific antibodies. Thus, their commitment to provide novel therapies to multiple myeloma patients, which is a disease with unmet needs, will be supported by this collaboration.
By Product/Modality
By Therapeutic Area/Indication
By Development Stage/Offering
By Manufacturing/Service Model
By Production Technology
By Region
February 2026
February 2026
February 2026
February 2026