February 2026

The global pharmaceutical contract manufacturing and research services market size was estimated at USD 281.68 billion in 2025 and is predicted to increase from USD 301.23 billion in 2026 to approximately USD 551.01 billion by 2035, expanding at a CAGR of 6.94% from 2026 to 2035.
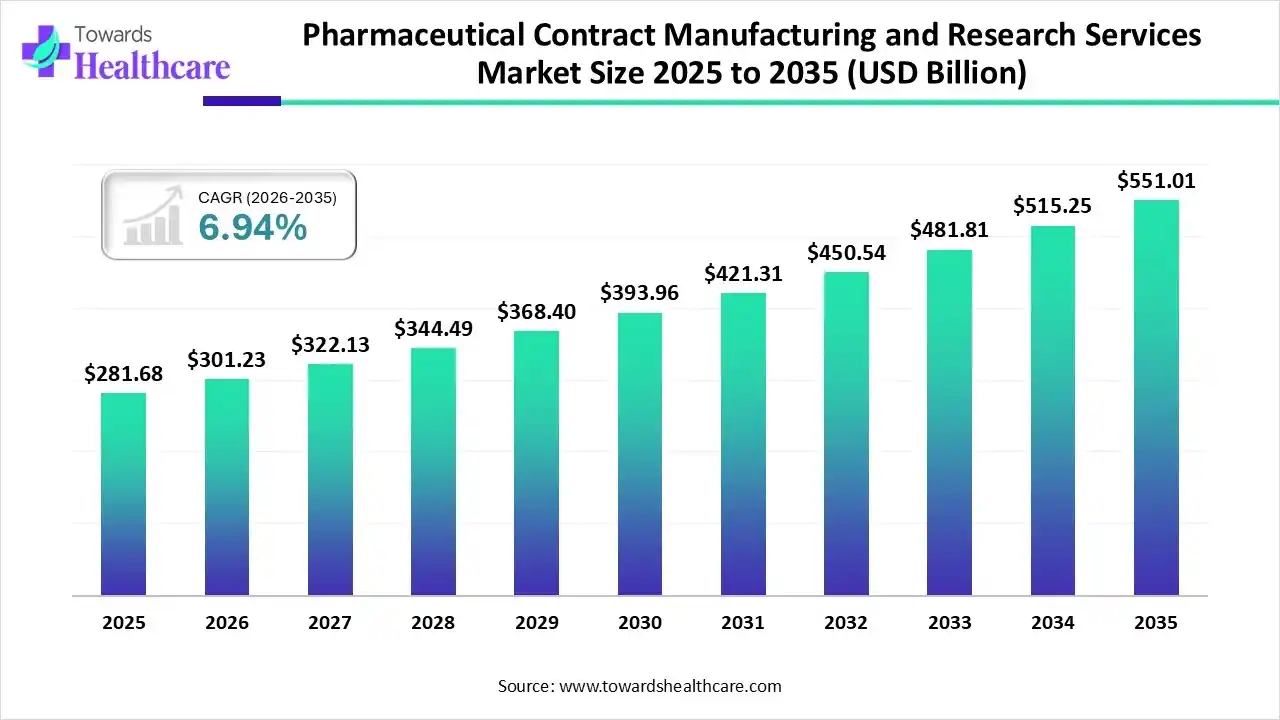
The growing advancement in drug discovery and development is increasing the use of pharmaceutical contract manufacturing and research services, where the increasing advanced therapies, AI integration, clinical trials, and new platform launches are also promoting the market growth.
The pharmaceutical contract manufacturing and research services market is driven by a growing shift towards end-to-end strategic collaboration, patent expiration, and increasing biologics. The pharmaceutical contract manufacturing and research services refer to the services provided to the pharmaceutical companies by specialized third-party companies. They offer a wide range of services supporting drug research, development, and manufacturing to the pharmaceutical companies.
The impact of AI on the pharmaceutical contract manufacturing and research services market is increasing as it helps in accelerating drug discovery and development, promoting the development of optimized products. It also analyses large datasets, provides insights, and reduces the chances of product failure. Its automated data processing and enhanced quality control detect defects in the systems and promote the development of consistent quality products.
The growing chronic disease burden globally is increasing the demand for cell & gene therapies, biologics, and biosimilars, which is increasing their development and innovations, driving the demand for pharmaceutical contract manufacturing and research services.
The growth in the R&D activities and production rates is increasing the outsourcing trends, leading to new collaborations for pharmaceutical contract manufacturing and research services, in order to focus on new drug candidates and reduce the cost associated with their development.
To boost efficiency and applications with end-to-end solutions and continuous manufacturing, new CRO-CDMO hybrid models are being launched along with the integration of automation and new digital tools.
| Key Elements | Scope |
| Market Size in 2026 | USD 301.23 Billion |
| Projected Market Size in 2035 | USD 551.01 Billion |
| CAGR (2026 - 2035) | 6.94% |
| Leading Region | Asia Pacific |
| Market Segmentation | By Service , By End-User, By Region |
| Top Key Players | Lonza Group, Thermo Fisher Scientific, Catalent, WuXi AppTec, IQVIA, Siegfried Holding, Boehringer Ingelheim, Recipharm, Samsung Biologics, Eurofins Scientific |
Which Service Type Segment Held the Dominating Share of the Market in 2025?
The manufacturing segment held the dominating share of the pharmaceutical contract manufacturing and research services market in 2025, due to continuous commercial-scale production. The growth in the demand for sophisticated equipment, GMP compliance, and expensive facilities increased the use of pharmaceutical contract manufacturing and research services. Advancements in biologics also increased their demand.
Research
The research segment is expected to show the highest growth during the upcoming years, due to growing drug discovery and development. Similarly, increasing innovations of gene therapies and biosimilars are also increasing the demand and use of pharmaceutical contract manufacturing and research services. The growing demand for specialized expertise and increasing outsourcing trends are also driving their acceptance rates.
What Made Big Pharma the Dominant Segment in the Market in 2025?
The big pharma segment contributed the biggest revenue share of the pharmaceutical contract manufacturing and research services market in 2025, due to growth in the R&D activities and clinical pipeline. This increased the use of pharmaceutical contract manufacturing and research services for specialized expertise and regulatory compliance. Moreover, growth in the drug production rates also increased the collaborations to leverage these services.
Contract Research Organizations
The contract research organizations segment is expected to witness rapid growth during the predicted time, due to growing outsourcing trends. The increasing advancements in the discovery and development of new molecules are also increasing the demand for pharmaceutical contract manufacturing and research services, where their affordability, regulatory expertise, and advanced technologies are also increasing their use.
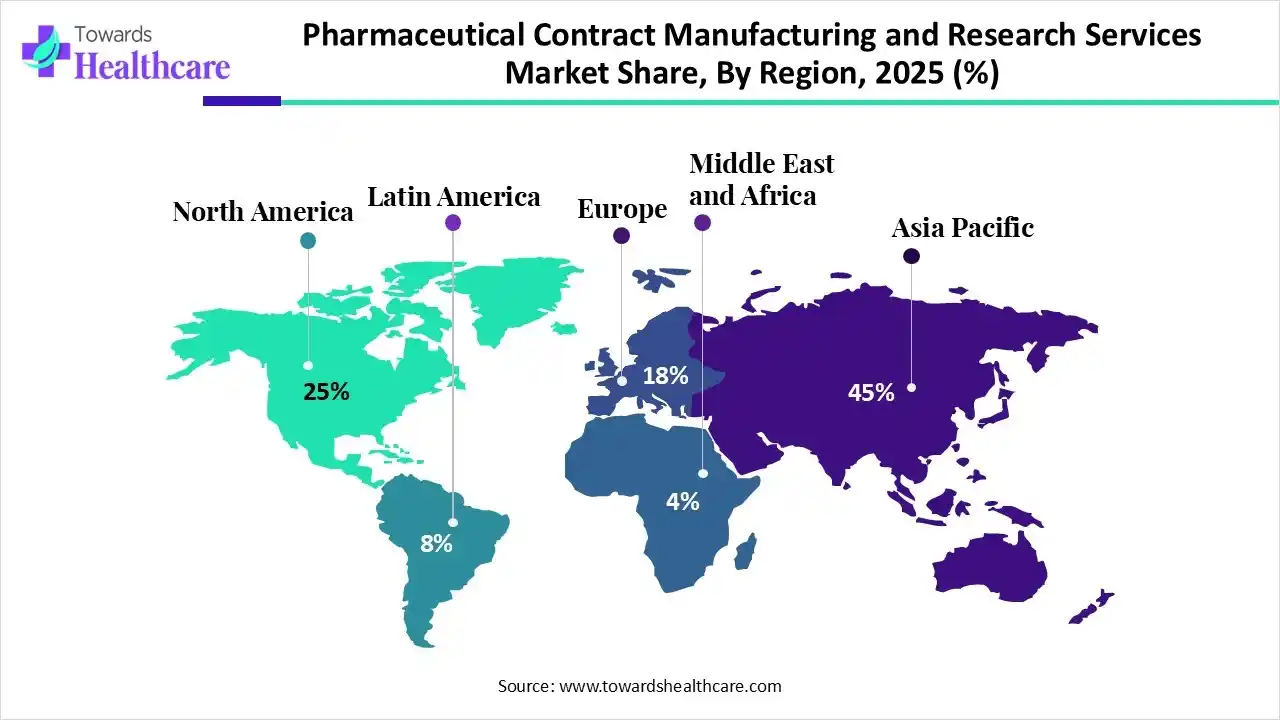
Asia Pacific dominated the pharmaceutical contract manufacturing and research services market in 2025, due to the rapid expansion of pharmaceutical and biotechnology companies, which is increasing the development of new products, encouraging the use of pharmaceutical contract manufacturing and research services. Growing outsourcing trends, disease burden, and government support are also enhancing the market growth.
India Market Trends
The growth in the CRO and CDMO ecosystem in India is increasing their collaboration to accelerate the development of various drug products. The growth in the generic product development and increasing skilled personnel are also increasing the use of pharmaceutical contract manufacturing and research services, where their affordability and quality controls are also promoting the acceptance rates.
North America is estimated to be the fastest-growing region in the pharmaceutical contract manufacturing and research services market during 2026-2035, due to growth in the R&D investments, which increased the R&D activities, driving the demand for pharmaceutical contract manufacturing and research services. The presence of well-developed pharmaceutical industries and startups also increased their use due to growing drug development advancements and clinical trials, which contributed to the market growth.
U.S. Market Trends
The U.S. consists of large pharmaceutical and biotech companies that are backed by R&D investment, which is accelerating drug discovery and development. Increasing demand for advanced therapies and personalized medication is also driving their innovations where stringent regulations are also increasing the use of pharmaceutical contract manufacturing and research services.
Europe is expected to grow significantly in the pharmaceutical contract manufacturing and research services market during the forecast period, due to the presence of advanced pharmaceutical industries, increasing the use of pharmaceutical contract manufacturing and research services due to growing drug development and innovations. Stringent regulations, growing clinical trials, and government investments are also promoting the market growth.
UK Market Trends
The presence of robust industries and R&D infrastructure in the UK is increasing the advancements in gene therapies, personalized medicines, and biologics, driving the demand for pharmaceutical contract manufacturing and research services. Growing clinical trials and government investments are also increasing their use, leading to new collaborations.

| Companies | Headquarters | Pharmaceutical Contract Manufacturing and Research Services |
| Lonza Group | Basel, Switzerland | Services for biologics, small molecule API and cell and gene therapies |
| Thermo Fisher Scientific | Waltham, U.S. | End-to-end solutions from drug substance manufacturing to commercial packaging and clinical research |
| Catalent | Somerset, U.S. | Offer advanced drug delivery technologies, sterile injectables, and oral dose formulation. |
| WuXi AppTec | Shanghai, China | Comprehensive “CRDMO” services, such as discovery chemistry, small molecule manufacturing, and preclinical research |
| IQVIA | Durham, U.S. | Extensive clinical trial management, data analytics, and commercialization services |
| Siegfried Holding | Zofingen, Switzerland | Drug substance and drug product manufacturing |
| Boehringer Ingelheim | Ingelheim, Germany | Process development to fill and finish services for biopharmaceuticals |
| Recipharm | Stockholm, Sweden | Manufacturing services for various dosage forms |
| Samsung Biologics | Incheon, South Korea | Large biopharmaceutical manufacturing services for mRNA and monoclonal antibody products |
| Eurofins Scientific | Luxembourg City, Luxembourg | Integrated drug discovery, development, and analytical testing services |
By Service
By End-User
By Region
February 2026
February 2026
February 2026
February 2026