January 2026

The quantum flex cell expansion system market is rapidly advancing on a scale, with expectations of accumulating hundreds of millions in revenue between 2026 and 2035. Market forecasts suggest robust development fueled by increased investments, innovation, and rising demand across various industries.
The quantum flex cell expansion system market is growing rapidly, as it offers an automated, scalable, and closed solution that can potentially increase the efficiency, consistency, and affordability of cell and gene therapy production compared to traditional physical processes.
Quantum flex enables modern therapy designers to complete their initial process advancement on the same platform that they’ll use for entire-scale production, and create a cell culture environment in which cells thrive. The bioreactor platform offers ideal conditions for cell expansion with built-in advanced software to simplify the deployment of protocols to numerous systems. The technology integrates hollow-fiber perfusion expertise, which offers a cell culture environment where cells have continuous access to fresh media, gas exchange, and waste removal, which ensures standard conditions for cell expansion.
Integration of AI-driven technology in the quantum flex cell expansion system market, which drives the growth of the market as the system's developed software contributions with Good Manufacturing Practices (cGMP) agreement through features such as operator authentication, fleet management, and batch records. This makes the high-quality, consistent information necessary for AI and machine learning submissions to modernize processes and ensure government compliance, which drives the growth of the market.
The Quantum Flex system is an automated, functionally closed system that will have augmented integration with other upstream and downstream processing skills
The system's design eases cGMP compliance, a significant feature as more cell therapies shift from clinical trials to commercialization
Advanced software topographies supporting cGMP compliance, consumer authentication, batch records, and fleet management are being improved to streamline government approval and ensure process consistency.
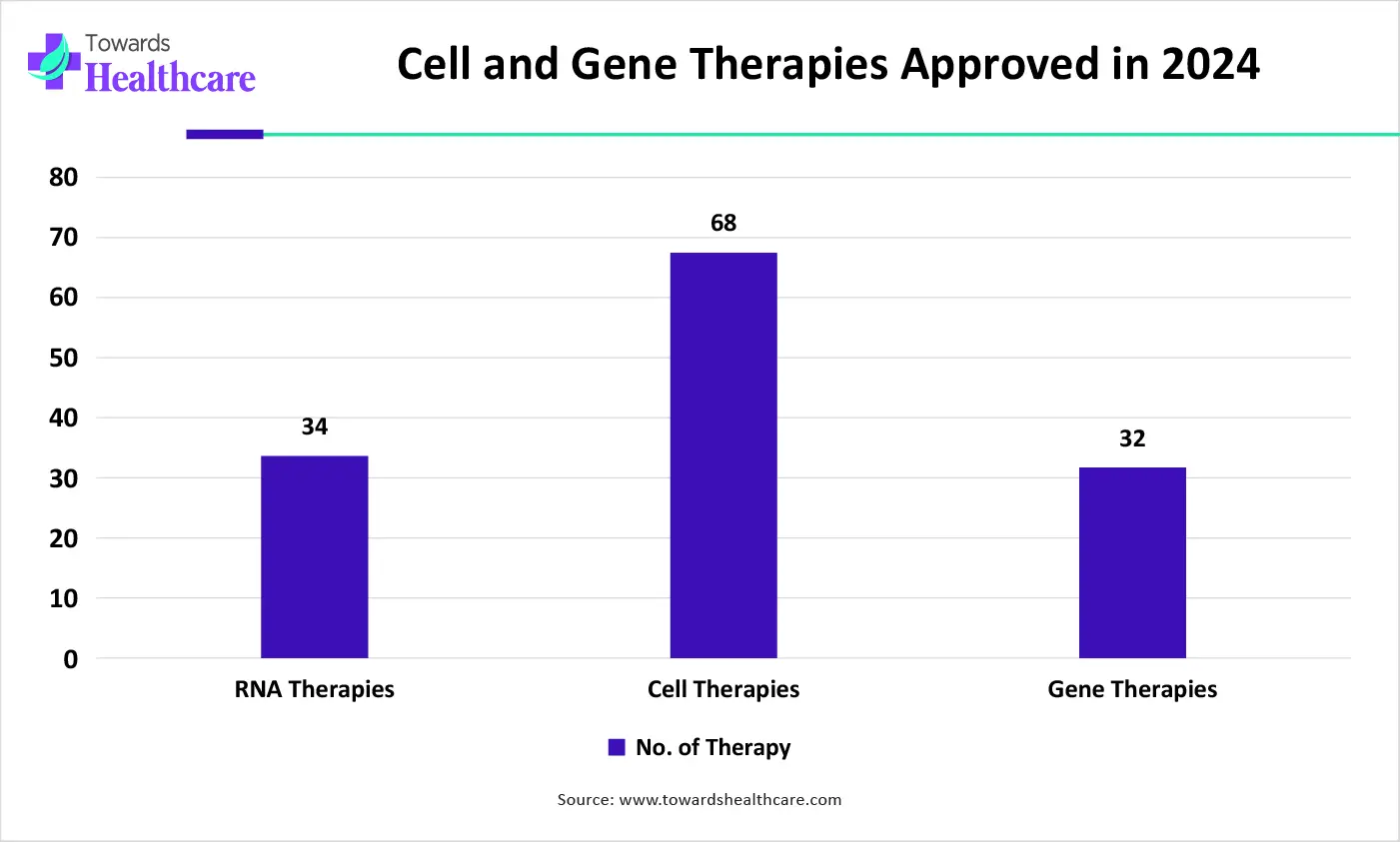
| Type of Therapy | No of Therapy |
| RNA Therapies | 34 |
| Cell therapies | 68 |
| Gene Therapies | 32 |
Which System Component Led the Quantum Flex Cell Expansion System in 2025?
In 2025, the disposable cell expansion sets segment held the dominant market, as it is single-use, pre-sterilized components are applicable for each batch, and the challenges of cross-contamination among the different cell lines or batches are virtually removed. Novel facilities need fewer heavy, fixed stainless steel substructures, leading to lower capital spending.
Software & Analytics Platform
Whereas the software & analytics platforms segment is the fastest growing in the market, as data analytics tools support an organization's process of numerous data sets, subsequent affordable and scalable protocols, which is nearly incredible on-site. Analytics platforms enable the collection and analysis of massive datasets from various sources, like bioreactors and culture circumstances.
Why did the Mesenchymal Stem Cells (MSCs) Segment Dominate the Market in 2025?
The mesenchymal stem cells (MSCs) segment captured the biggest revenue share of the quantum flex cell expansion system in 2025, as mesenchymal stem cells are used to show corresponding expansion kinetics during a 7-day expansion procedure on both the small and standard bioreactors, revealing efficacy differences.
T-Cells
Whereas the T-cells segment is the fastest growing in the market, as quantum platform joins T cell activation, lentiviral vector transduction, and CAR-T expansion to modernize the production technology. Advanced software enables remote monitoring, fleet management, and automated data gathering.
Why is the Biopharma & Cell Therapy Companies Segment Dominant in the Market?
In 2025, the biopharma & cell therapy companies segment held the dominating share of the market, as the quantum flex system provides the opportunity to use fewer cells (<30 million T cells or more than 5 million MSCs), allowing for simple process advancement before switching to the larger-sized bioreactors needed for large-scale production. It is trendy and broadly applied in academia, in healthcare settings, and in industry.
CDMOs/Cell Therapy Manufacturing Partners
Whereas the CDMOs/cell therapy manufacturing partners segment is the fastest growing in the market, as it provides significant benefits for CDMOs and cell therapy manufacturers by offering an automated, functionally closed platform that improves efficiency, scalability, and product quality, while lowering expenses and manufacturing challenges.
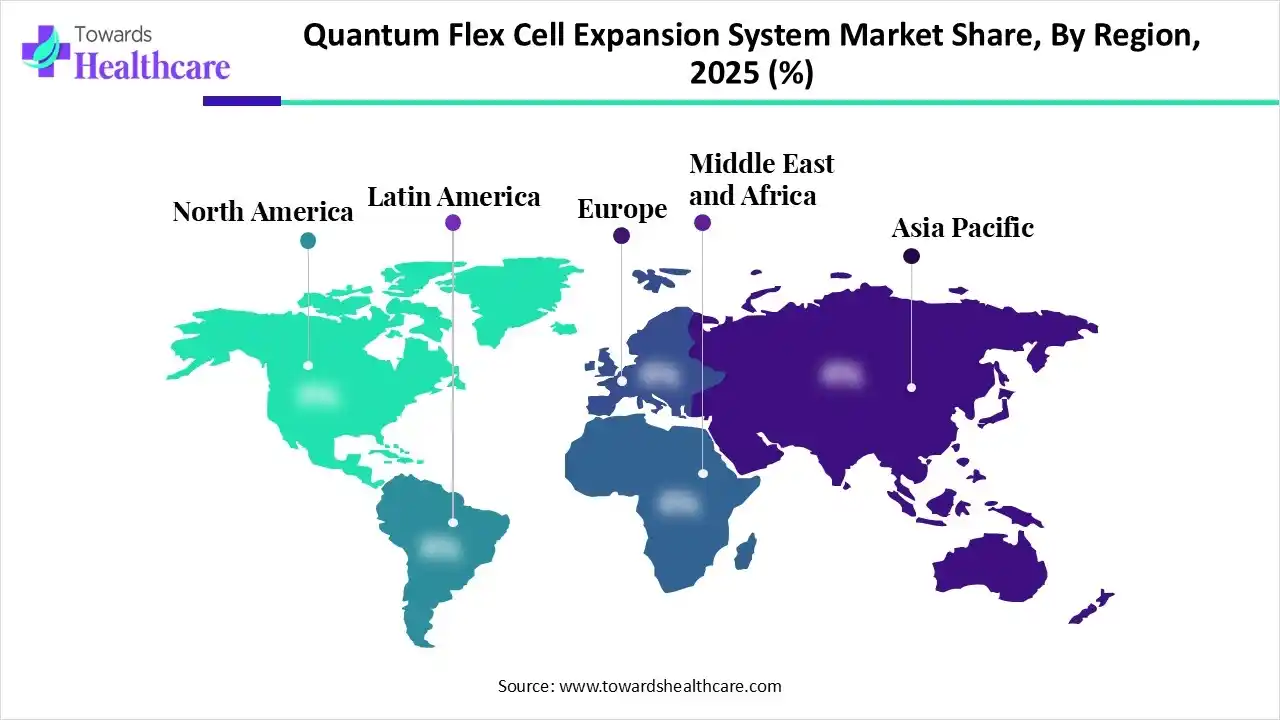
In 2025, North America led the quantum flex cell expansion system market, driven by the strong presence of leading biopharmaceutical companies, significant investments in cell-based research, and a high adoption rate of advanced technologies. The presence of major players Thermo Fisher Scientific, Becton, Dickinson and Company, and Terumo BCT has a strong presence and is headquartered in the United States, further contributing to the region's dominance, which drives the growth of the market.
For Instance,
In the U.S., Quantum Flex is an automated, functionally closed system intended to lower physical labor, reduce contamination challenges, and streamline the production process from process development to commercial production. Mechanization of bioreactors and expansion platforms is a main trend driving the market for cell expansion tools.
Asia Pacific is set to experience rapid growth in the quantum flex cell expansion system, driven by this region's increasing regulatory support and investments for stem cell and regenerative medicine research, with strategic initiatives in countries such as China, India, and Japan. An increasing demand for developed therapies and biopharmaceutical manufacturing is driven by the increasing prevalence of chronic diseases and an aging population, which contributes to the growth of the market.
For Instance,
India is experiencing a surge in local pharmaceutical and biotechnology organizations developing and commercializing cellular therapies. The country has evolved as a hub for Global Capability Centers (GCCs) in R&D and invention. Growing government funding and initiatives in stem cell research and regenerative medicine speed up the demand for advanced cell expansion platforms, which drives the growth of the market.
Europe is experiencing significant growth in the medical market, driven by the strong development in the cell and gene therapy (CGT) sector that drives the adoption of automated systems like the Quantum Flex Cell Expansion System, manufactured by Terumo Blood and Cell Technologies. Strong spending in biotechnology R&D and supportive government frameworks, which drive the growth of the market.
The UK is a significant player in the wider cell and gene therapy (CGT) market, and its development is linked to the adoption of advanced manufacturing platforms, such as the Quantum Flex Cell Expansion System, which is driving market growth.

| Company | Headquarters | Latest Update |
| Terumo Blood and Cell Technologies (Terumo BCT). | Lakewood, Colorado, USA | In November 2025, researchers at the University of Chicago in the US completed all three critical steps in the manufacturing of T-cell receptor (TCR) therapy (TCR-T). |
| FUJIFILM Irvine Scientific | United states | Terumo Blood and Cell Technologies (Terumo BCT) and FUJIFILM Irvine Scientific have announced a strategic collaboration to help accelerate T cell expansion. |
| Thermo Fisher Scientific | United states | In November 2025, Thermo Fisher Scientific Deepens Investment in Asia's Biopharma Ecosystem with Expansion of Bioprocess Design Centers |
| Merck KGaA | Germany | It provides various solutions that can be used in conjunction with such systems, including cell culture media and other bioprocessing solutions. |
| BD | United states | It streamlines a process that typically involves fragmented, manual workflows. |
| Sartorius | In September 2025, Sartorius will expand production of key components for cell and gene therapies in France. | |
| Miltenyi Biotec | Germany | In June 2025, Miltenyi Biotec India and the Biotechnology Industry Research Assistance Council (BIRAC) signed a letter of intent for a collaboration aimed at enhancing India’s capabilities in cell and gene therapy through capacity building. |
By System Component
By Cell Type
By End-User
By Region
January 2026
January 2026
January 2026
January 2026