February 2026

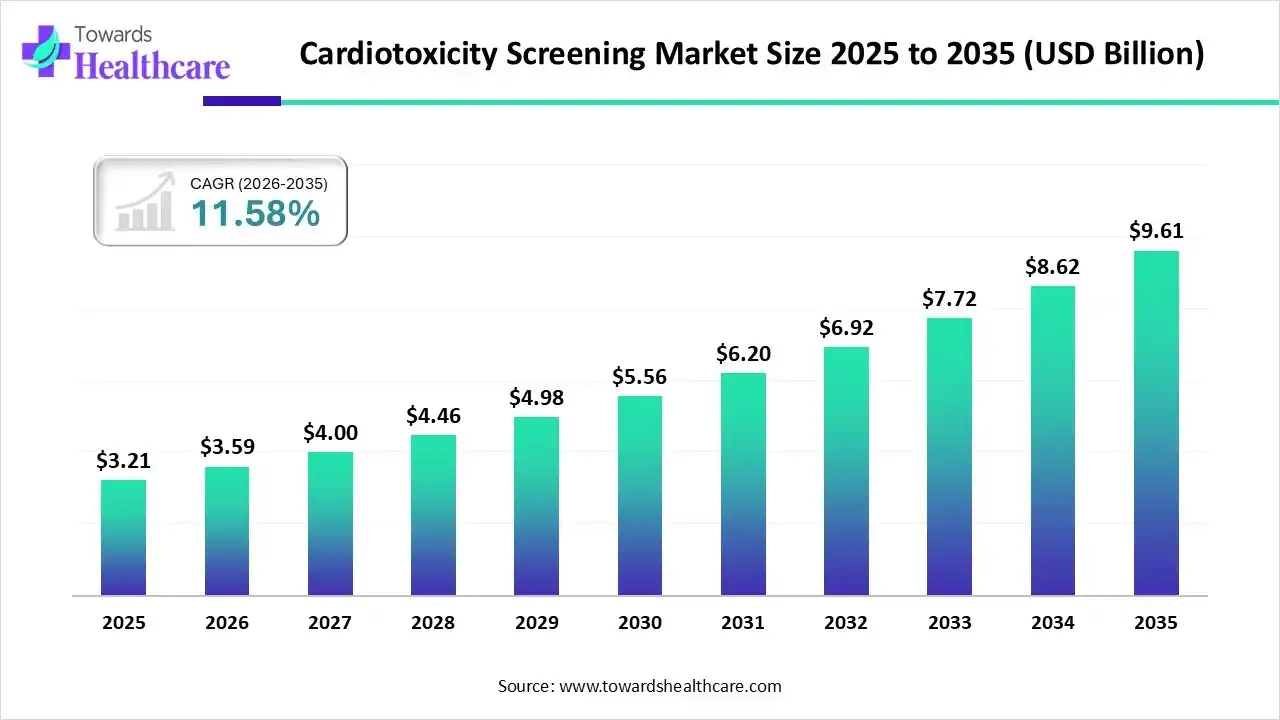
The global cardiotoxicity screening market size was estimated at USD 3.21 billion in 2025 and is predicted to increase from USD 3.59 billion in 2026 to approximately USD 9.61 billion by 2035, expanding at a CAGR of 11.58% from 2026 to 2035.
Across the globe, rising cases of cardiac diseases and the expanding need for early phase detection are propelling the demand for advanced cardiotoxicity screening solutions. Besides this, several companies are leveraging 3D biomarker models and modern AI platforms to meet the stringent regulatory guidelines.
The application of a vital safety assessment process for finding drugs or chemicals affecting the heart, which results in arrhythmia and minimal contraction, is referred to as cardiotoxicity screening. This cardiotoxicity screening market is driven by the growing number of cancer cases, which need cardiotoxic therapies, with the incorporation of stricter regulatory safety guidelines. Extensive advances encompass Zebrafish models, which have a major role in the detection of myocardial apoptosis & heart rate modification. Also, researchers are fostering microfluidic heart-on-a-chip solutions, such as BioFlux.
Specifically, AI has a substantial role in early detection, such as AI supports the analysis of standard 12-lead ECGs to find subclinical left ventricular dysfunction, & also detects risks weeks before traditional echocardiograms show a lowering in ejection fraction, like the AI-CTRCD model. Moreover, at AHA 2025, AI-SCREEN-CA software was rolled out, which utilises deep learning on echocardiographic loops to determine cardiac amyloidosis (CA) early, detecting 17% of suspected, high-risk incidences.
Recently, the firms validated certain newer biomarker panels for prior detection of cardiotoxicity in cancer patients, for which individualized risk scoring systems, like PREVENT equations, are being employed to identify high-risk individuals.
The market is focusing on using "organ-on-a-chip" technology to model interactions between diverse cell types, including cardiomyocytes, endothelial cells, fibroblasts & vascular flow.
Researchers are evolving assays to emphasise non-electrophysiological pathways, such as mitochondrial dysfunction, metabolic perturbations, and structural damage.
| Key Elements | Scope |
| Market Size in 2026 | USD 3.59 Billion |
| Projected Market Size in 2035 | USD 9.61 Billion |
| CAGR (2026 - 2035) | 11.58% |
| Leading Region | North America |
| Market Segmentation | By Assay/Test Type, By Modality/Approach, By Product and Service Type, By End User, By Region |
| Top Key Players | Charles River Laboratories, Labcorp/Covance, Eurofins Scientific, WuXi AppTec, Evotec, ICON plc, Certara, Axion BioSystems, Multi-Channel Systems (MCS) |
Which Assay/Test Type Registered Dominance in the Cardiotoxicity Screening Market in 2025?
In 2025, the hERG (Kv11.1) binding and patch-clamp assays segment led the market. Its significant benefit is the estimation of the risk of drug-induced QT interval prolongation and Torsades de Pointes (TdP), which leads to fatal ventricular arrhythmia. A current aim at modeling dynamic, state-dependent binding of drug-hERG interactions is impacting the overall progression. Recently, XGBoost-ISE Mapping launched, which integrates XGBoost with Isometric Stratified Ensemble (ISE) mapping to facilitate strong predictions for hERG liability.
Multi-Electrode Array (MEA) with iPSC-Cardiomyocytes
The multi-electrode array (MEA) with iPSC-cardiomyocytes segment will witness rapid growth. This is an immersive, non-invasive, and high-throughput approach used to assess cardiotoxicity in drug development. Whereas iPSCs enable the generation of patient-specific, or even disease-specific, cardiomyocytes, such as those with Long QT syndrome. Alongside, a latest study showed closed-loop "traveling wave" (TW) systems unified with MEAs, which utilise rapid electrical pacing to bolster the sarcomeric and functional maturation of the cells.
How did the In Vitro (Cell and Tissue-Based) Segment Lead the Market in 2025?
The in vitro (cell and tissue-based) segment dominated the cardiotoxicity screening market in 2025. It is mainly propelled by the rising requirement for more precise, affordable, and ethical options to animal testing in drug development. Ongoing innovations include 3D bio-printed cardiac tissues, which use decellularized extracellular matrix (dECM) hydrogels as bio-ink, for mimicking the complex 3D anisotropic structure of the human heart.
In Silico/Computational (Simulation, PBPK-QT Models)
The in silico/computational (simulation, PBPK-QT models) segment will expand rapidly. Particular adoption of in-silico screening enables the detection of adverse effects during the discovery stage, which prevents expenditures on toxic molecules & clinical trials. However, validated PBPK models are assisting in establishing "virtual populations" for future anticipation of drug exposure and improvement in dosing regimens for actual clinical trials. Recently, the QSP-PBPK-TD platform for anti-tumor drugs was developed by connecting the PBPK model with Quantitative Systems Pharmacology (QSP) and Toxicology Dynamics (TD).
Which Product and Service Type Dominated the Cardiotoxicity Screening Market in 2025?
In 2025, the assay kits, reagents, and ready-to-use cells (iPSC-cardiomyocytes, dyes, media) segment registered dominance in the market. Kits, like the EarlyTox Cardiotoxicity Kit, support measuring intracellular calcium flux, mitochondrial membrane potential, and general cytotoxicity. This segment also encompasses calcium-sensitive dyes, like FLIPR Calcium 5, & specialized media, such as MyoMax. Additionally, axocells confirmed against the Comprehensive in vitro Pro-arrhythmia Assay (CiPA) compound panel for high-throughput electrophysiology & calcium imaging.
Contract Testing Services/CRO Screening Programs
Moreover, the contract testing services/CRO screening programs segment is anticipated to expand fastest. It is primarily fueled by CRO’s inexpensive, specialized, high-throughput in vitro and in vivo screening services for lowering drug attrition rates. They are emphasizing the use of sophisticated human-induced pluripotent stem cell-derived cardiomyocyte (hiPSC-CM) technologies, 3D cardiac organoids, and AI-powered predictive modeling to raise safety assessments.
Which End User Led the Cardiotoxicity Screening Market in 2025?
The pharmaceutical and biotechnology companies (in-house screening) segment was dominant in the market. Players, like Novartis, Pfizer, Inc., etc., are highly involved in the development of cardioprotective drugs and assessing existing drugs for preventing heart damage. Nowadays, companies are promoting modern machine learning, including graph neural networks trained on the FDA’s Drug-Induced Cardiotoxicity Risk (DICTRank) dataset.
CROs/Safety and Toxicology Service Providers
The CROs/safety and toxicology service providers segment will witness rapid expansion. Specifically, they explore in vitro & in vivo testing, and also, advanced cardiac imaging and high-throughput screening tools. Alongside, CROs are widely leveraging the Comprehensive in vitro Proarrhythmia Assay (CiPA), mandating strong, early-phase cardiac safety assessments to avoid late-stage failures. The latest approach is "Cardiotox Screen" panels for evaluating both structural and functional endpoints from a single cell population.
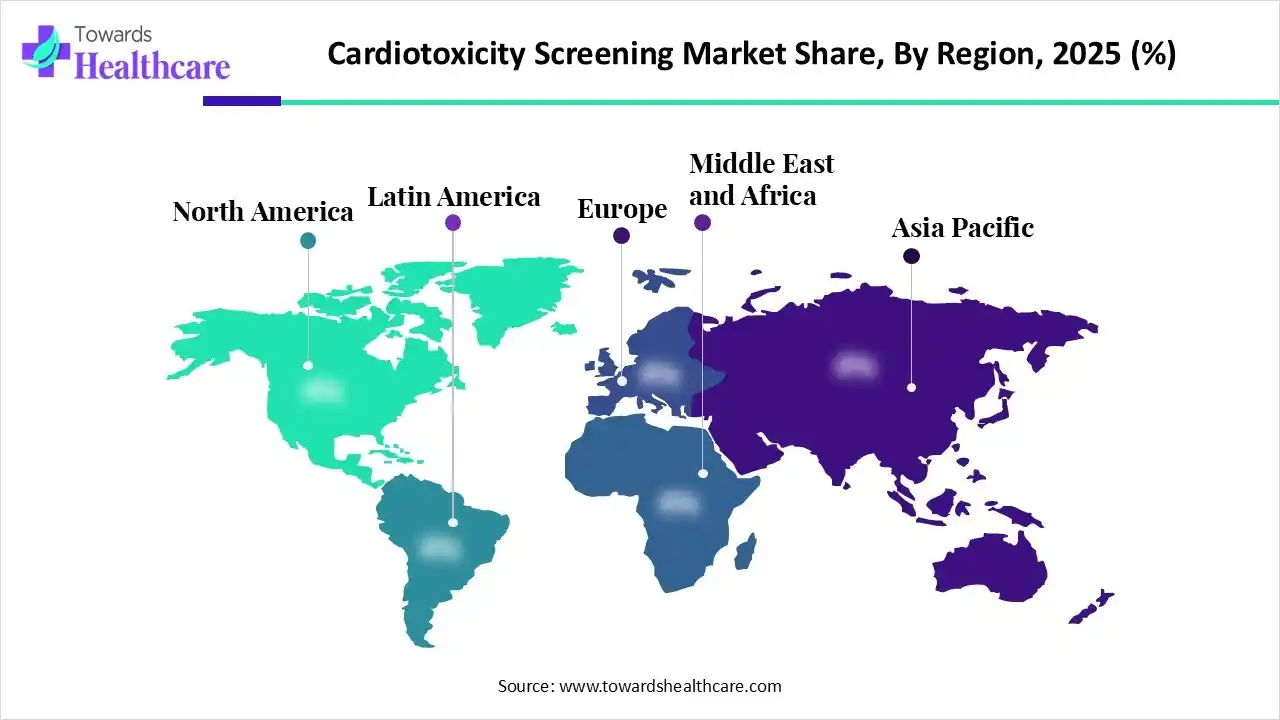
North America captured the biggest share of the cardiotoxicity screening market, due to the stricter regulatory safety requirements, a rise in oncology-related cardiac risks, and increased R&D investment in drug development. Also, the region is spurring novel projects, especially the Jackson Laboratory (JAX), which is fostering "CARDIOVERSE," by using stem cell-based systems (iPSC-CMs) and AI to develop virtual hearts, or "digital twins" of cardiac tissue.
U.S. Market Trends
The U.S. was a major contributor in this regional market, as recently the FDA initiated formalizing its plan to phase out mandatory animal testing for some drugs, which supports manufacturers in submitting safety data derived from NAMs. The U.S. is increasingly bolstering ML solutions to Electrocardiograms (ECGs) for detecting subclinical structural heart diseases & estimating prospective heart failure.
Asia Pacific is predicted to witness the fastest growth in the cardiotoxicity screening market. A prominent catalyst is continuous investment in drug discovery, mainly in China, Japan, and India, which is driven by the need for early-stage in-vitro cardiotoxicity testing. However, Singapore & across Asian researchers are broadly adopting 3D human heart tissue, which is developed from stem cells, for new oncology and cardiovascular drug evolutions.
India Market Trends
India is emphasizing preventive cardiac care through youth screening campaigns, which include mandatory early, regular screening for at-risk individuals, like lipid profiles, ECG, and stress tests, even for young adults. Also, they are stepping towards uniting cardiovascular specialists into cancer care teams for tracking chemotherapy-induced cardiac damage.
Europe is anticipated to expand significantly in the market, as it is focusing on the widespread adoption of wearable devices, like the Garmin Venu SQ and Polar H10 sensor. These devices are encouraging consistent monitoring of older, multimorbid breast cancer patients to find cardiotoxicity before it becomes clinically evident.
UK Market Trends
Moreover, the UK researchers found a set of 29 intracellular ceramides, which act as a predictive metabolic signature for structural cardiotoxicity, and further enable early detection of damage in cardiac microtissues.

| Company | Description |
| Charles River Laboratories | This usually explores a complete set of cardiotoxicity screening services through its Safety Pharmacology & Discovery Services divisions. |
| Labcorp/Covance | Its offerings include in vitro & in-vivo cardiovascular safety, biomarkers, and specialized testing. |
| Eurofins Scientific | Their portfolio assists regulatory compliance (FDA/ICH) and early-stage drug discovery. |
| WuXi AppTec | This facilitates ion channel assessments, stem cell-derived cardiomyocyte platforms, and specialized imaging techniques. |
| Evotec | Its offering comprises integration of human-relevant cell models with advanced analytical technologies. |
| ICON plc | This has explored a "one-stop shop" solution, which combines clinical pharmacology units (CPUs) with an experienced cardiac safety core lab. |
| Certara | A firm emphasizes in silico modeling, simulation, and predictive analytics. |
| Simulations Plus | This provides in silico tools and services for cardiotoxicity screening, especially through its ADMET Predictor & GastroPlus platforms. |
| Axion BioSystems | Its offering focuses on its Maestro multielectrode array (MEA) platform. |
| Multi-Channel Systems (MCS) | This explores the entire set of electrophysiology tools for in vitro and in vivo cardiotoxicity screening. |
By Assay/Test Type
By Modality/Approach
By Product and Service Type
By End User
By Region
February 2026
February 2026
February 2026
February 2026