October 2025

The worldwide medtech labeling automation market is experiencing significant expansion, with projections indicating a revenue increase reaching several hundred million dollars by the end of the forecast period, spanning 2025 to 2034. This growth is driven by emerging trends and strong demand across key sectors.
The global medtech labeling automation market is experiencing significant expansion, with increased adoption of automated labeling systems in robust and numerous pharmaceutical, medical device, and healthcare areas. As well as the market is fueled by its major advantages in various sectors, including affordability, boosted effectiveness, and ultimate growth in productivity. Whereas, in the future, several opportunities will arise, such as customized labelling approaches to meet out specific demands of various stakeholders, like healthcare providers and patients, with accelerating digitalization in life science companies.
The medtech labeling automation market refers to software and hardware systems that automate the creation, management, and printing of regulatory-compliant, multilingual, and traceable labels for medical devices, diagnostics, and in vitro diagnostics (IVDs). These solutions are essential to meet UDI (Unique Device Identification), EU MDR/IVDR, FDA, and global labeling standards. Automation enhances efficiency, reduces labeling errors, and ensures label version control and traceability across complex global MedTech supply chains. Market growth is fueled by increasing regulatory complexity, product diversity, and digital transformation in MedTech manufacturing.
In 2025, artificial intelligence is playing a crucial role in the market, due to the rising complexity of medical devices, which need highly advanced labelling requirements, as well as enhanced demand for rapid product development cycles with effective labelling operations. In these cases, AI algorithms help in boosting efficiency, precision, and compliance with regulations. Moreover, AI-driven solutions can assist in automating tasks such as label creation, verification, and tracking, with minimized errors and expenditure linked with human approaches.
Accelerating Applications of Automated Systems
In the medtech labeling automation market, developing drivers are in numerous companies such as pharmaceuticals and medical device production need effective and precise labeling with raised pace. Along with this, the advantages of these emerging automated systems like affordability, minimized manual force, resulting in optimized overall efficiency. As well as these approaches are boosting the reduction of downtime, progressing productivity. Furthermore, the contribution of advanced technologies, including AI and ML, is supporting automated defect detection, improving label placement, and estimation of maintenance for labelling equipment.
Escalating Prior Investment and Technical Limitations
Widely involved challenges, such as major initial investment in hardware, software, and infrastructure, are creating a vital obstacle for smaller-scale medical device producers with restricted expenditure provision. Alongside, unified automated labelling approaches possesses existing production lines, particularly legacy systems are developing another technical barrier in the medtech labeling automation market.
Broader Adoption of Personalized Labelling and Digital Labelling
According to research, in prospects, several important opportunities may arise, including tailored labelling approaches to meet out specific demands of various stakeholders, like healthcare providers and patients. As well as, by using these automated techniques allows for more efficient monitoring and tracing of medical devices throughout the supply chain for robust serialization and medical coding. A major opportunity in the medtech labeling automation market is the rising adoption of cloud platforms, which provide access to labeling systems for players of all sizes, including smaller processes, and allow them to handle difficult regulations and shifting provider requirements.
By component, the software segment dominated the market in 2024. Due to enhanced automation in regulated industries, especially in the healthcare area, medical devices and pharmaceuticals have adopted automation to optimize effectiveness and precision in labelling systems is fueling the segment growth. Also, raising demand for accurate and compliant labeling is critical in medtech industries is driven by the use of these developing softwares.
Whereas, the services segment will show the fastest growth, with the emergence of novel technologies, including AI, machine learning, and RFID are generating numerous opportunities for service providers to enable specialized solutions for data management, label design, and system integration. Basically, the segment is propelled by rising geriatric population, rising case of chronic diseases and boosting healthcare expenses.
The hybrid deployment segment was dominant in the market due to combined factors like increased balance between the management of on-premise systems and the scalability and affordability of cloud-based solutions. Also, it is fueled by its important benefit, such as maintainance of sensitive data on-premise while grasping cloud approach for other operations, are preventing some data related risks.
On the other hand, the cloud-based segment is predicted to show rapid expansion, with the incorporation of many factors, including growing data volume, the requirement for scalability and availability, and the step for quick, more effective labelling systems. Also, this segment provides accelerated collaboration, decreased infrastructure spending, and strong compliance with regulations, which is making them attractive to medtech producers.
In 2024, the UDI compliance & regulatory labeling segment led the medtech labeling automation market. In case of medical device regulations, like adoption of UDI requirements are complex and this complexity is fueling the need for sophisticated and standardized labelling approaches to confirm compliance around the various regions and skip potential delays, fines, or recalls. Also, UDI is important for boosting patient safety by optimizing the traceability of medical devices in their lifecycle.
During 2025-2034, the label version control & audit readiness segment will expand rapidly, due to rising stringent regulations in medtech companies related to labelling, like requirements for accurate information, traceability, and audit trails. For this, automated systems assist to achieve the demand by capturing label versions, tracking modifications, and offering audit-ready documentation.
The medical device manufacturers segment registered dominance in the market in 2024. Primarily, the segment is fueled by growing complexity in modern medical devices, which needs more detailed and precise labelling, for this automation can manage complexity with minimal risk of errors. Important advancements in automation, robotics, and machine-to-machine connectivity are generating novel choices for medical device manufacturers to simplify their labelling systems and optimize effectiveness.
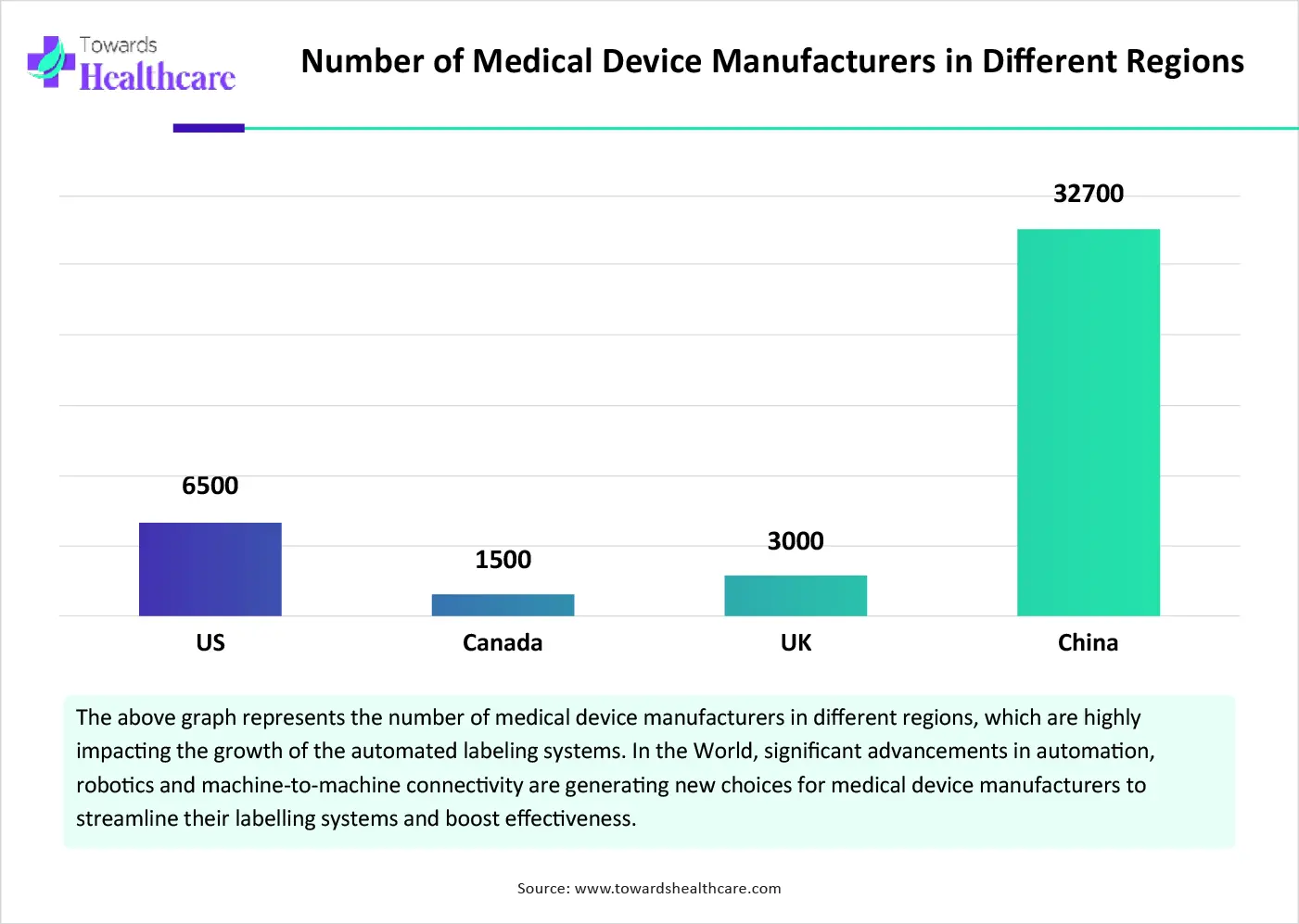
By end user, the packaging & supply chain teams segment is estimated to grow rapidly in the coming years. The segment is driven by the escalating adoption of smart packaging techniques, like RFID and NFC or QR codes, which allow real-time tracing and product standardization, fueling the demand for automated labelling systems. Along with this, these approaches use robotic palletizing and automated guided vehicles (AGVs), developing a fully automated and improved workflow.
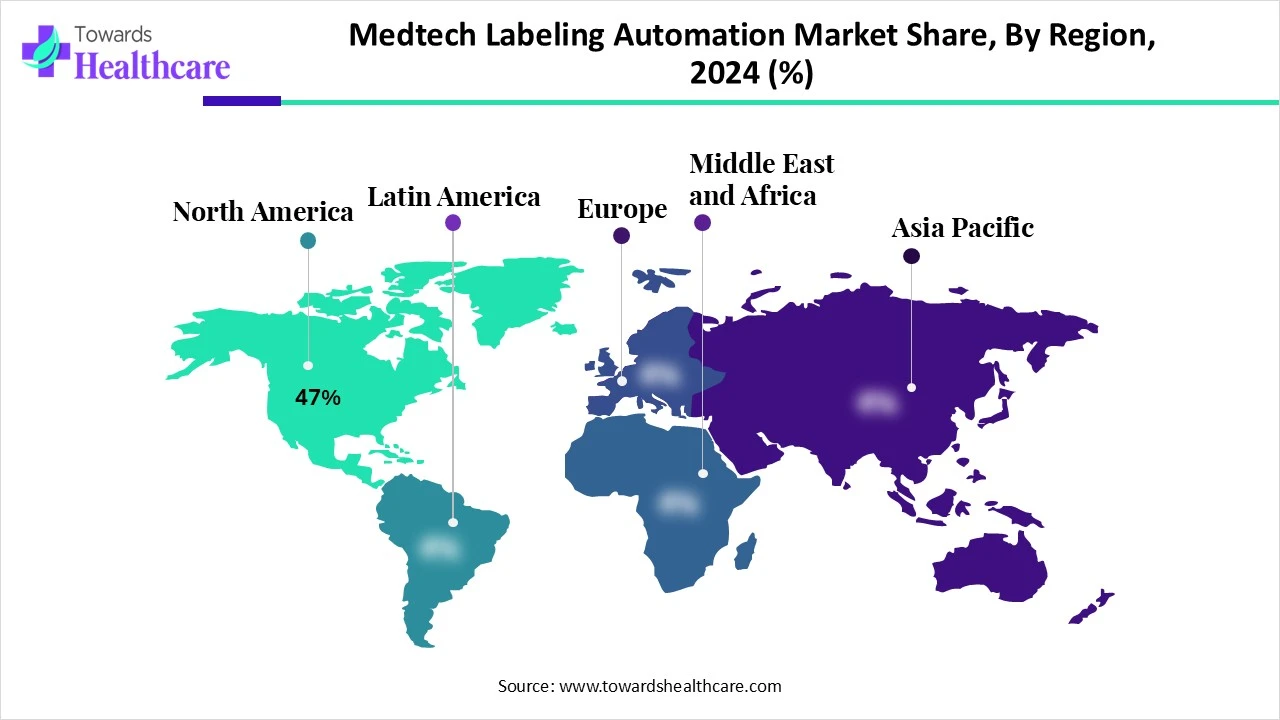
In the respective market, North America held the biggest revenue share by 47% in 2024. Due to early UDI adoption, FDA mandates, and a mature digital infrastructure are supported in the market expansion is supported. Moreover, this region is emphasizing environmental awareness is boosting sustainable labeling solutions, including eco-friendly packaging materials and minimal waste strategies, which offer more efficient processes through automation. Also, the region is widely adopting AI, machine learning, and robotics, which ultimately is assisting demand for automated labelling systems.
Mainly, the US FDA regulations for the pharmaceutical and medical device sectors need accurate and traceable labelling approaches. Besides this, the US is highly step towards digital health platforms and mobile applications, which are impelling need for digital labeling solutions, like QR codes and AR with sophisticated processes for strong implementation.
For this market,
Canada is increasingly focused on rising healthcare spending and the development of healthcare infrastructure, which are accelerating the demand for automated labelling solutions in hospitals and diagnostic centers. Moreover, Canada’s progressing pharmaceutical companies necessaries effective labeling systems to meet production demands and regulatory frameworks. Also, in Canada, increased labeling accuracy is vital for patient safety, particularly in medication dispensing and management.
In the studied years, the Asia Pacific is predicted to be the fastest-growing in the market, due to a major impacting factor being the expansion in medtech hubs across China, India, South Korea, and Singapore. As well as boosting pharmaceutical production and export activities, with accelerating demand for precise labeling to confirm drug safety, and the accelerating incidence of chronic conditions are resulting in widespread pharmaceutical adoption.
The Indian government is encouraging healthcare digitalization and infrastructure enhancement, allied with initiatives boosting smart industries and digital infrastructure with further market expansion. India possess strong base for pharmaceutical companies are widely demanding for automation with raised productivity, and efficient products.
For instance,
China is greatly focused on the development of the e-commerce sector, which is assisting a surge in demand for packaging solutions, such as automated labeling machines, to manage the enhanced volume of shipped goods. As well as, China aims to increase effectiveness, minimize labour expenses, and optimize accuracy in labelling processes is fostering producers to adopt these novel sophisticated systems, mainly in the pharmaceutical and medical device industries.
In 2025 and the coming era, Europe is growing notably in the medtech labeling automation market. Highly impacting factors are European stringent regulations relevant to product safety, traceability, and serialization are fostering for advanced labelling solutions. Besides this, Europe has promising growth in medical research and development is propelling demand for specialized labeling approaches for clinical trial materials.
The UK’s major investments in healthcare infrastructure and digitization, alongside novel creations in robotics, machine vision, and AI, are boosting the potential of automated labeling systems with their raised adoption. As well as in the development of diverse biologics and biosimilars, which requires unique and specialized labeling approaches, are fueling the market growth.
Germany is a leading country in Europe for packaging automation and medical device, with a robust engineering ecosystem and major investments in modernization. Also, this region is shifting interest towards the development of eco-friendly labeling choices, such as by using the biodegradable and recyclable materials, which is broadly impacting the respective market growth.

By Component
By Deployment Mode
By Application
By End User
By Region
October 2025
November 2025
November 2025
November 2025