503A U.S. Compounding Pharmacies Market Companies - You Must Explore!

- Triangle Compounding
- Fagron
- B. Braun SE
- Pencol Specialty Pharmacy
- Vertisis Custom Pharmacy
- Optum Inc
- Pavilion Compounding Pharmacy, LLC.
- Village Compounding Pharmacy
- McGuff Compounding Pharmacy
- Wedgewood Pharmacy
503A U.S. Compounding Pharmacies Market Overview
The FDA has elected 503A compounding pharmacies as those pharmacies that compound medicines as per prescriptions particular to patients and must adhere to USP and other regulations as mandated by state boards of pharmacy. 503A facilities are not allowed to manufacture in large batches and they are meant to produce and dispense for home use only. The government has employed some regulations for compounders to strictly follow while compounding pharmacies. Regulations suggested for 503A include USP <795> and <797> along with state board of pharmacy regulations.
Such type of compounding can be done in community pharmacies, hospital pharmacies, and in physicians offices. The United States Pharmacopoeia (USP) offers environmental standards for 503A compounding pharmacies in the United States, recommending that compounded medications be produced in specified spaces that are suitably constructed to facilitate the sterile or nonsterile processes, which comprises offering correct storage for those preparations and the right circumstances (e.g., regulated temperature, light, ventilation, security, and moisture).
Market Growth
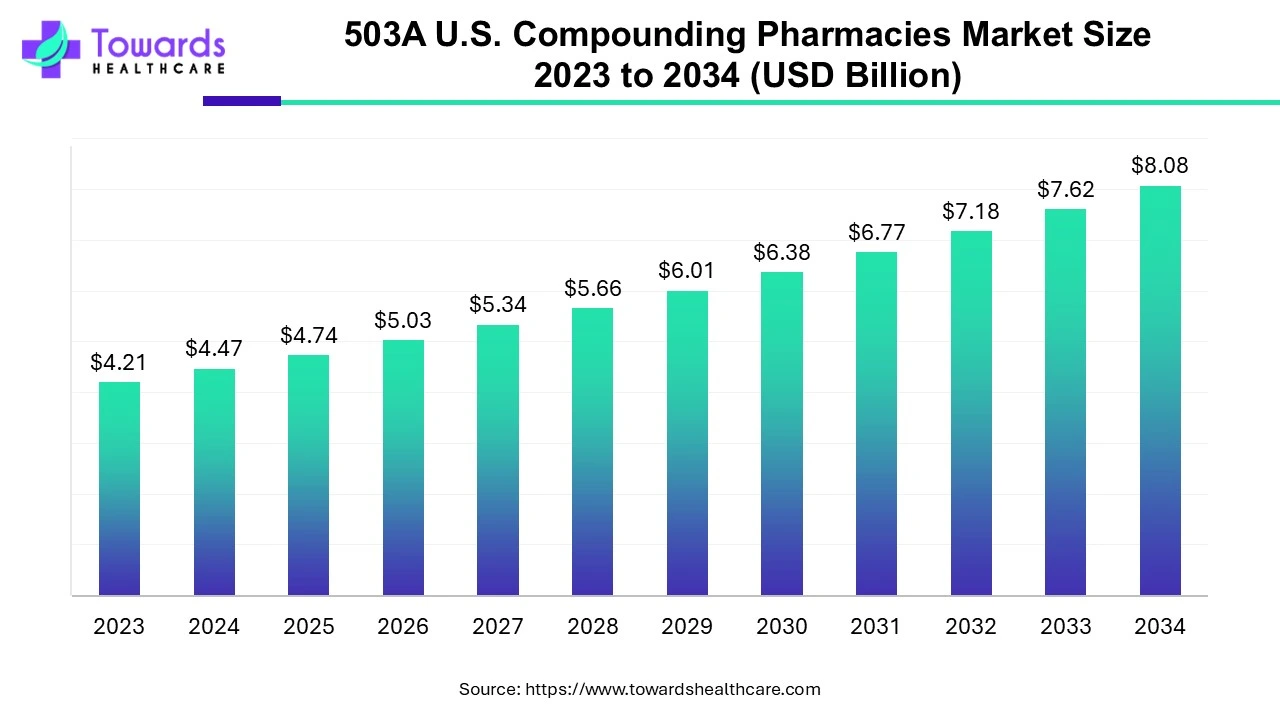
The 503A U.S. compounding pharmacies market is projected to reach USD 8.08 billion by 2034, growing from USD 4.74 billion in 2025, at a CAGR of 6.11% during the forecast period from 2025 to 2034, increasing demand for personalized, convenient and accessible care.
503A U.S. Compounding Pharmacies Market Trends
- In February 2025, Empower announced the launch of its new corporate logo, marking a significant step in the company’s evolution as a unified and forward-thinking organization. The company announced the unification of 503A compounding pharmacy and 503B outsourcing facilities under a single brand.
- In January 2025, Revelation Pharma announced the acquisition of Cascade Specialty Pharmacy to integrate ENT and Animal Health compounding into Revelation Pharma’s growing portfolio.
- In December 2024, LRockRx Compounding Pharmacy, a new 503A compounding pharmacy, announced the opening of its new facility in Little Rock to serve patients, healthcare providers, veterinarians, and the local community in Arkansas. The new facility is estimated to create 100 jobs with an average salary of $100,000 annually.
503A Compounding Pharmacy Market Outlook
- Industry Growth Overview: The market is experiencing robust growth, driven by the growing need for personalized medicines and the rising prevalence of chronic disorders. The increasing population results in drug shortages, potentiating the need for a compounding pharmacy.
- Major Investors: Compounding pharmacies are attracting funding from private equity and venture capital firms, due to high drug shortages and a surge in demand for niche formulations. This is followed by the flexibility in opaque pricing mechanisms by pharmaceutical companies.
- Startup Ecosystem: The startup ecosystem for the 503A U.S. compounding pharmacy market is maturing with favorable funding policies and the increasing awareness of compounding pharmacy benefits.
Strategic Initiatives
- In March 2025, Empower Pharmacy expanded its operations by opening a second 503B outsourcing facility in Houston, Texas. This new site significantly enhanced its ability to produce sterile compounded medications, increasing capacity by over 150%. The facility is equipped with cutting-edge automation and robotic systems, allowing for greater efficiency, precision, and scalability in sterile compounding processes.
- In February 2025, Fagron introduced an innovative software platform designed specifically for 503A compounding pharmacies. This new system combines compounding workflow management with tools for tracking patient outcomes, aiming to improve both operational efficiency and clinical results. By streamlining the compounding process and offering real-time data on patient responses, the platform helps 503A pharmacies enhance quality assurance, compliance, and overall patient care.
- In November 2024, Bruno Onwukwe, PharmD candidate, and Celeste Zizzamia, PharmD, explain the key differences between 503A compounding pharmacies and 503B outsourcing facilities under the FDA’s Drug Quality and Security Act (DQSA). With drug shortages and updates to USP 797 and USP 800, many 503A pharmacies are now looking to collaborate with 503B facilities for sterile and hazardous compounding needs. The article reviews Section 503B and offers tips on how 503A pharmacies can evaluate and choose the right 503B partner.
Latest Announcement by Industry Leaders
Shawn Hodges, CEO of Revelation Pharma, commented that they are delighted to cultivate a compounding pharmacy network built upon a standard of excellence. He also said that their dedication to excellence is further demonstrated by the acquisition of Cascade Specialty Pharmacy.
Recent Developments
- In September 2024, a coalition of 503A and 503B compounding pharmacies introduced a national wholesale platform aimed at healthcare providers. This initiative simplifies the procurement process for specialized medications and compounded formulations, offering a more efficient and reliable ordering experience.
- In September 2024, Wedgewood added molnupiravir to its formulary. Initially created for treating viral infections in humans, molnupiravir is now being used to combat a frequently fatal disease that impacts approximately 15,000 cats in the U.S. annually, raising serious concerns among veterinarians and pet owners.
Collaborate with our experts to explore the 503A U.S. Compounding Pharmacies Market at sales@towardshealthcare.com
Keypoints
- Company Overview
- Locations Subsidiaries/Geographic reach
- Key Executives
- Company Financials
- Patents registered
- SWOT Analysis
- Applications Catered
- Strategic collaborations
- Recent Developments
- Competitive Benchmarking

