MariTide (AMG 133 / maridebart cafraglutide) is Amgen’s investigational, first-in-class bispecific molecule that combines GLP-1 receptor agonism with GIP-receptor antagonism using a peptide-antibody conjugate. It is designed for monthly (or less frequent) dosing to treat obesity and related metabolic disease.
Amgen’s Role and Responsibilities
As a leading biopharmaceutical company, Amgen manages the full lifecycle of MariTide, from research to potential commercialization:
- Research & Development: Conduct preclinical and multi-phase clinical trials to ensure efficacy, safety, and optimal dosing.
- Clinical Program Leadership: Manage the MARITIME Phase-3 program, collaborating with CROs and investigators to generate robust data.
- Regulatory Submissions: Interact with agencies like FDA and EMA to prepare filings, address queries, and define post-marketing commitments.
- Manufacturing & Supply: Scale complex peptide-antibody conjugates for commercial production while maintaining quality.
- Commercial Strategy & Market Access: Develop pricing, reimbursement strategies, and health economics models to facilitate adoption.
- Medical Affairs & KOL Engagement: Educate clinicians, support guideline updates, and provide data to investigators.
- Pharmacovigilance: Monitor safety post-launch and generate real-world evidence on efficacy and adherence.
- Competitive Positioning: Differentiate MariTide against market leaders like semaglutide and tirzepatide.
Latest Announcements and Innovations
- Phase-2 Results: MariTide demonstrated up to 20% average weight loss in obesity cohorts and up to 17% in patients with type 2 diabetes, along with a reduction in HbA1c by up to 2.2 percentage points.
- Safety Profile: Discontinuation rates due to adverse effects were 11% overall, primarily gastrointestinal, with the highest dose group experiencing 27% dropout.
- Mechanistic Innovation: The bispecific design aims to enhance weight loss durability and reduce rebound weight gain.
- Phase-3 MARITIME Program: Two pivotal 72-week studies, MARITIME-1 and MARITIME-2, are underway to confirm efficacy and safety in broader populations.
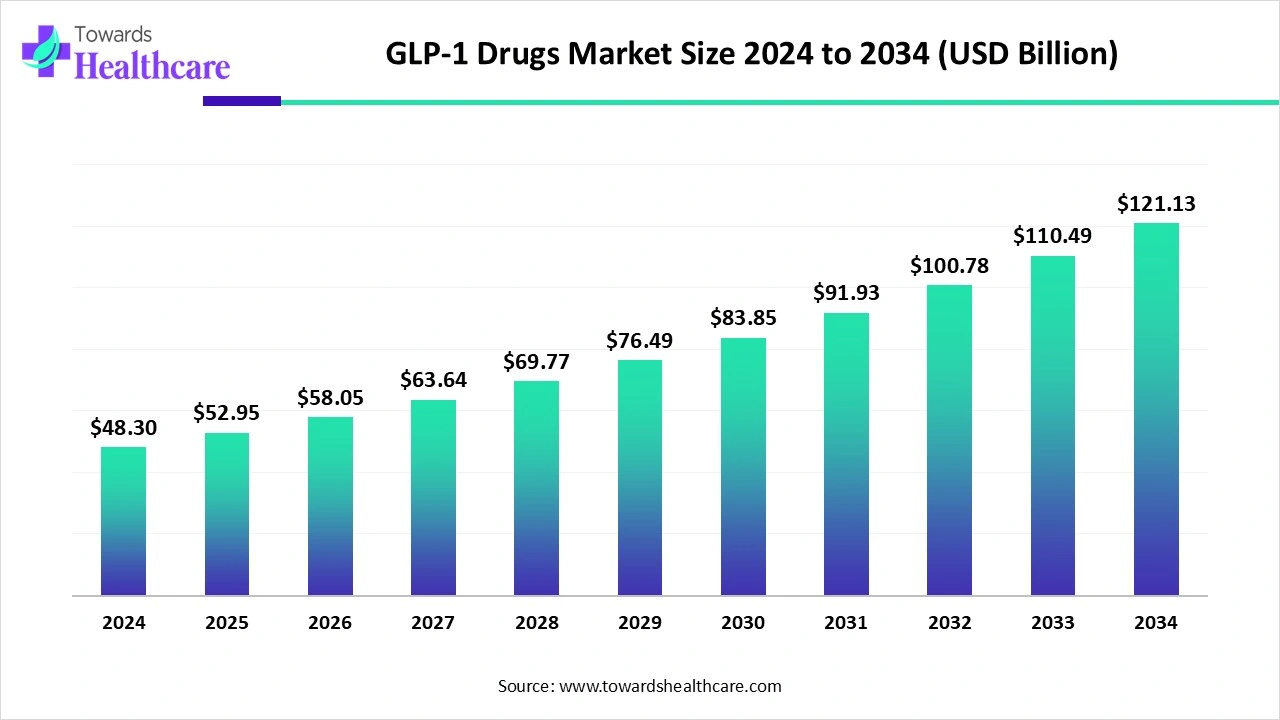
GLP-1 Drugs Market Potential in 2025
The global GLP-1 drugs market size was valued at US$ 48.3 billion in 2024 and is projected to reach US$ 52.95 billion by 2025. By the end of 2034, it is expected to grow US$ 121.13 billion, growing steadily at a CAGR of 9.63%.
MariTide enters a booming obesity drug market with strong growth projections. Its monthly dosing and novel mechanism could capture meaningful market share, especially if Phase-3 trials confirm superior or differentiated efficacy. However, adoption will depend on:
- Comparative efficacy against semaglutide, tirzepatide, and other GLP-1 therapies.
- Payer coverage and reimbursement policies.
- Manufacturing scalability and cost management.
Challenges and Considerations
- Competition: Novo Nordisk and Eli Lilly dominate the GLP-1 market, with emerging oral candidates increasing pressure.
- Comparative Effectiveness: Payers and clinicians will require strong evidence of superiority or unique benefits.
- Reimbursement: Cost-effectiveness will influence adoption, particularly in markets with evolving coverage.
- Adherence & Retention: Long-term patient adherence and management of side effects are critical.
- Manufacturing Complexity: Peptide-antibody conjugates pose challenges for large-scale production.
- Regulatory Scrutiny: Safety and long-term outcomes will be closely monitored.
Keypoints
- Company Overview
- Locations Subsidiaries/Geographic reach
- Key Executives
- Company Financials
- Patents registered
- SWOT Analysis
- Applications Catered
- Strategic collaborations
- Recent Developments
- Competitive Benchmarking
