Let's Explore the Leading Companies in the Ankylosing Spondylitis Market
Unlocking the Spine: Innovations Reshaping the AS Landscape
The ankylosing spondylitis market is experiencing robust growth, driven by the increasing prevalence of spondyloarthropathies, early diagnosis initiatives, rising adoption of biologics, and the growing demand for targeted immunotherapies. It includes pharmaceuticals, biologics, biosimilars, diagnostics, monitoring tools, and supportive care services used for the treatment and long-term management of AS, a chronic, progressive inflammatory disease primarily affecting the axial skeleton. The market covers TNF inhibitors, IL-17 inhibitors, JAK inhibitors, NSAIDs, DMARDs, physical therapy solutions, diagnostic imaging, and disease monitoring technologies.
Market Growth
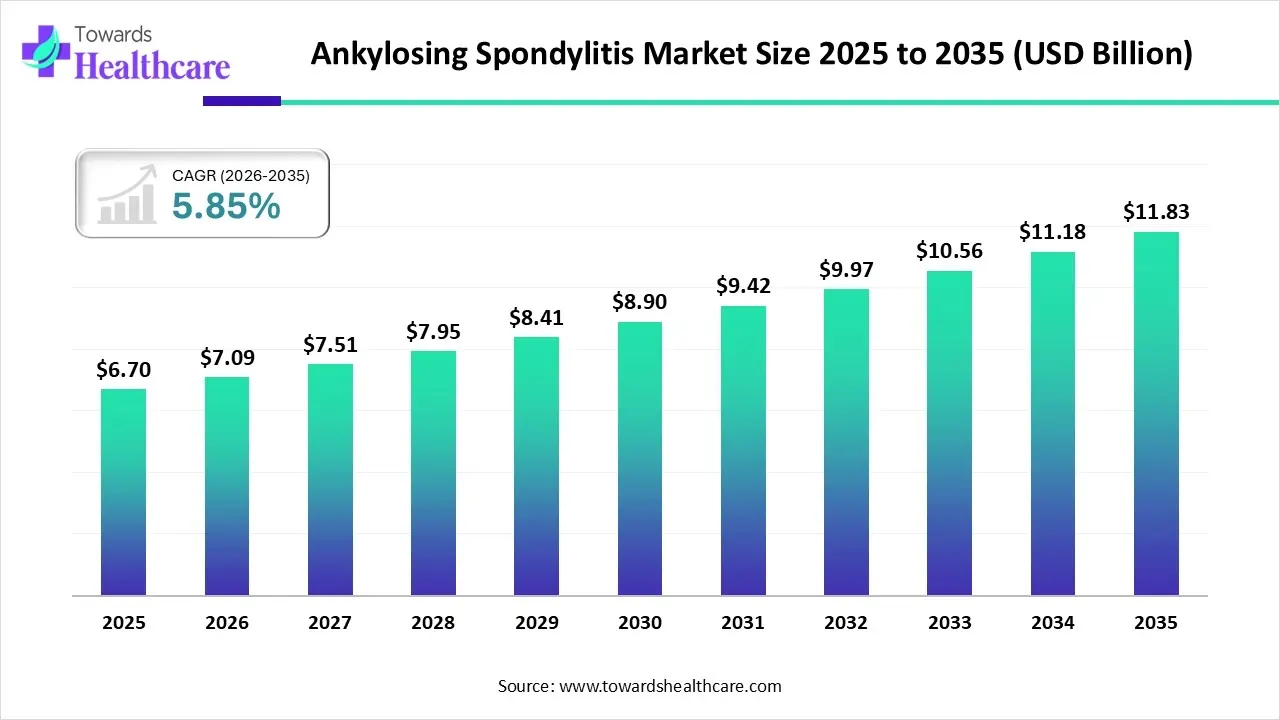
The global ankylosing spondylitis market size is calculated at USD 6.7 billion in 2025, grew to USD 7.09 billion in 2026, and is projected to reach around USD 11.83 billion by 2035. The market is expanding at a CAGR of 5.85% between 2026 and 2035.
Ankylosing Spondylitis Market Outlook
- Industry Growth Overview: The market is expected to grow rapidly, driven by the increasing development of personalized medicines and innovative diagnostics. Advances in cell therapy, particularly mesenchymal stem cells (MSCs) and CAR-T cell therapy, demonstrate promise in addressing limitations.
- Startup Ecosystem: The startup ecosystem is maturing, driven by significant growth in venture capital investments and the rising adoption of advanced technologies. Hillstar Bio, Trinity Biotech, and NeoDx Biotech Labs are some startups involved in the treatment and diagnosis of AS.
- Major Investors: Several government and private organizations provide funding to pharma and biotech companies, assisting in the development of innovative immunotherapies, stem cells, and in vitro diagnostics. Funding also allows healthcare organizations to adopt advanced tools for AS diagnosis and treatment.
Top Companies & Their Offerings in the Ankylosing Spondylitis Market
1. Pfizer, Inc. (New York, United States)
2. Sandoz Group AG (Basel, Switzerland)
-
Provides Hyrimoz, used alone or with non-biologic DMARDs for the treatment of AS.
-
Revenue (2024): $10.4 billion.
3. Novartis AG (Basel, Switzerland)
-
Offers Cosentyx, the first and only interleukin-17A inhibitor approved for adults with AS.
-
Revenue (2024): $11.9 billion.
4. Augurex Life Sciences Corp. (Canada)
-
Developed SPINEstat, a first-of-its-kind multiplex biomarker assay for diagnosing and managing AS.
-
Revenue (2024): Not available / Not disclosed.
5. Eli Lilly and Company (Indiana, United States)
Company Landscape
1. Novartis AG
Company Overview:
- Novartis is a global healthcare leader focused on innovative medicines, generics, and biosimilars, with a strong presence in immunology, oncology, and neuroscience.
Corporate Information:
- Headquarters: Basel, Switzerland
- Year Founded: 1996 (merger of Ciba-Geigy and Sandoz)
- Ownership Type: Public (Aktiengesellschaft)
History and Background:
- Formed by the merger of Ciba-Geigy and Sandoz in 1996 to create a diversified pharma group. The company was later structured into divisions like Innovative Medicines and Sandoz.
Key Milestones/Timeline:
- 1996: Merger of Ciba-Geigy and Sandoz to form Novartis.
- 2015: FDA originally approved Cosentyx (secukinumab) for psoriasis; later expanded.
- 2024: European Commission granted landmark approval for Cosentyx in ankylosing spondylitis.
- 2025: $23 B U.S. investment announced for R&D and manufacturing.
Business Overview:
- Novartis develops, manufactures, and markets a broad range of pharmaceutical products, including patented medicines, generics, and biosimilars.
Business Segments/Divisions:
- Innovative Medicines (pharma and oncology)
- Sandoz (generics & biosimilars)
- Corporate / Holding functions
Geographic Presence:
- Global operations in ~140 countries, with ~78,000 employees.
Key Offerings (in AS):
- Cosentyx (secukinumab), IL-17A inhibitor for ankylosing spondylitis.
End-Use Industries Served:
- Primarily pharmaceutical/healthcare — rheumatology, immunology, inflammation.
Key Developments and Strategic Initiatives:
- U.S. facility expansion: $23 B to build 10 plants, including a research hub in San Diego.
- Collaboration with Schrödinger (computational drug discovery) for next-gen molecules.
Mergers & Acquisitions:
- In 2025, Novartis acquired Anthos Therapeutics (up to $3.1 B) to bolster its cardiovascular pipeline.
Partnerships & Collaborations:
- Multi-year collaboration with Schrödinger valued up to $2.5 B to integrate computational discovery.
Product Launches/Innovations:
- Continued life cycle management of Cosentyx, including IV formulations in other indications.
Capacity Expansions/Investments:
- Major U.S. manufacturing and R&D build-out as part of $23 B plan.
Regulatory Approvals:
- FDA approvals for Cosentyx in ankylosing spondylitis and psoriatic arthritis.
- European Commission approval for AS indication.
Distribution Channel Strategy:
- Novartis distributes globally via a network of subsidiaries, wholesalers, and specialty pharma channels.
Technological Capabilities/R&D Focus:
- Strong focus on biologics, computational discovery, precision medicine, and novel modalities.
Core Technologies/Patents:
- Monoclonal antibodies (IL-17) such as secukinumab.
- Proprietary platforms in computational drug design via Schrödinger collaboration.
Research & Development Infrastructure:
- Multinational R&D centers (e.g., Basel, U.S.)
- Global clinical trial network for immunology indications, including AS.
Innovation Focus Areas:
- Next-gen immunology therapies
- Computational drug discovery
- Biologics and biosimilars
Competitive Positioning:
- Strengths & Differentiators: First mover with IL-17A inhibitor in AS; strong global footprint; deep R&D.
- Market Presence & Ecosystem Role: Major biologics innovator; sets benchmark in IL-17 class.
SWOT Analysis:
- Strengths: Broad global reach; innovation pipeline; deep immunology expertise.
- Weaknesses: High manufacturing costs; patent expiry risk.
- Opportunities: Expand Cosentyx into more markets/indications; computational drug AI.
- Threats: Biosimilar competition; regulatory risk; pricing pressures.
Recent News & Updates:
- 2025: Announced $23 B investment in U.S. manufacturing/R&D.
- 2025: Collaborated with Schrödinger for computational drug discovery.
Press Releases:
- European Commission approval for Cosentyx in AS.
- FDA approval for AS/PsA indications.
Industry Recognitions/Awards:
- Recognized for R&D leadership and innovation in immunology by multiple industry observers (per annual reports).
2. AbbVie Inc.
Company Overview:
- AbbVie is a global biopharmaceutical company focused on immunology, oncology, neuroscience, and other specialty medicines.
Corporate Information:
- Headquarters: North Chicago, Illinois, USA
- Year Founded: 2013 (spin-off from Abbott Laboratories)
- Ownership Type: Public
History and Background:
- AbbVie was created in 2013 when Abbott Laboratories split its research-based business. It has built a strong immunology franchise around Humira and newer therapies.
Key Milestones/Timeline:
- 2013: AbbVie spun off from Abbott Laboratories.
- 2016–2020s: Humira dominated its revenue, then biosimilar competition grew.
- 2024–2025: Shift toward newer immunology assets – Rinvoq, Skyrizi.
Business Overview:
- AbbVie develops, manufactures, and markets prescription drugs, especially in immunology, oncology, and other specialty areas.
Business Segments/Divisions:
- Immunology
- Oncology
- Neuroscience
- Aesthetics & Women’s Health
Geographic Presence:
- Global presence, with operations in the U.S., Europe, Asia, and emerging markets.
Key Offerings (in AS):
- Humira (adalimumab), a TNF-alpha inhibitor widely used in ankylosing spondylitis.
- End-Use Industries Served:
- Healthcare/Pharmaceuticals, specifically for autoimmune diseases, rheumatology, and chronic inflammatory conditions.
Key Developments and Strategic Initiatives:
- Transitioning focus from Humira to next-gen immunology drugs.
- Negotiated biosimilar strategies and market access adjustments.
Mergers & Acquisitions:
- 2024: Acquired ImmunoGen for US$10.1 B to strengthen oncology pipeline.
- 2024: Bought Cerevel Therapeutics (~US$8.7 B) to boost neuroscience.
Partnerships & Collaborations:
- AbbVie has research collaborations across academia and biotech; its focus is on immunology, neuroscience, and oncology.
Product Launches/Innovations:
- Actively growing newer immunology products like Skyrizi and Rinvoq to reduce dependency on Humira.
Capacity Expansions/Investments:
- AbbVie is investing in manufacturing and pipeline to support next-generation biologics and small molecules.
Regulatory Approvals:
- Humira is approved for ankylosing spondylitis. AbbVie’s newer immunology drugs continue to get regulatory traction.
Distribution Channel Strategy:
- Uses global commercial networks, specialty pharmacies, hospital channels, and reimbursement partnerships.
Technological Capabilities/R&D Focus:
- Strong R&D in antibody engineering, immunology, small molecule drugs, and biosimilars.
Core Technologies/Patents:
- TNF-alpha biologics (Humira)
- Small molecules (JAK inhibitors like Rinvoq)
- IL-23 / IL-17 + novel mechanisms
Research & Development Infrastructure:
- Worldwide clinical trial presence; immunology labs; advanced analytics for biologic development.
Innovation Focus Areas:
- Next-gen immunology (beyond TNF)
- Biologics and small-molecule hybrid therapies
- Precision immune medicine
Competitive Positioning:
- Strengths & Differentiators: Strong legacy (Humira), deep immunology pipeline, global reach.
- Market Presence & Ecosystem Role: Key legacy biologics provider; pivoting to sustainable growth.
SWOT Analysis:
- Strengths: Established brand, broad immunology expertise; strong cash flow.
- Weaknesses: Patent expiry risk on Humira; biosimilar pressure.
- Opportunities: Expand newer drugs (Rinvoq, Skyrizi) into more indications, including AS.
- Threats: Biosimilar competition; regulatory pricing pressure; shifting payer policies.
Recent News & Updates:
- 2025: Q3 earnings beat, driven by Skyrizi (+47%) and Rinvoq (+35%) growth.
- 2024: Cigna announced the removal of Humira from some reimbursement lists in favor of biosimilars.
Press Releases:
- Public communications about immunology pipeline transformation and biosimilar strategy.
- Investor releases on earnings growth driven by newer immunology therapies.
Industry Recognitions/Awards:
- AbbVie is regularly highlighted as a leader in immunology R&D and commercial execution in specialty pharma circles.
Recent Developments in the Ankylosing Spondylitis Market
- In July 2025, Biocon Biologics Ltd. announced the launch of Nepexto, a biosimilar of the reference product Enbrel (Etanercept), in the Australian market. Biocon collaborated with Generic Health to deliver the drug in Australia, broadening patient access to affordable treatment options in the country.
- In April 2025, Biocad, the Russian National Research Medical University of Pirogov, and the Institute of Bioorganic Chemistry developed Seniprutug, a domestic drug for the treatment of AS. The drug will benefit patients from 45 Russian regions, which can relieve symptoms and stop its progression.
Collaborate with our experts to explore the Ankylosing Spondylitis Market at sales@towardshealthcare.com

