
The sector devoted to the creation, manufacturing, and marketing of vaccinations intended to prevent dengue disease is known as the dengue vaccine market. Another significant driver of market expansion is the growing funding and assistance provided by governmental and non-governmental organizations for the development and dissemination of dengue vaccines. Increased healthcare resources and public-private partnerships are being used to hasten the release of vaccines in dengue-endemic regions.
In the WHO Region of Europe (EURO), the Regions of the Americas (PAHO), South-East Asia and West Pacific Regions (SEA RO and WPRO, respectively), the Eastern Mediterranean WHO Region (EMRO), and Africa, 101 countries and territories have reported more than 4 million dengue cases and more than 2500 dengue-related deaths since the start of 2025.
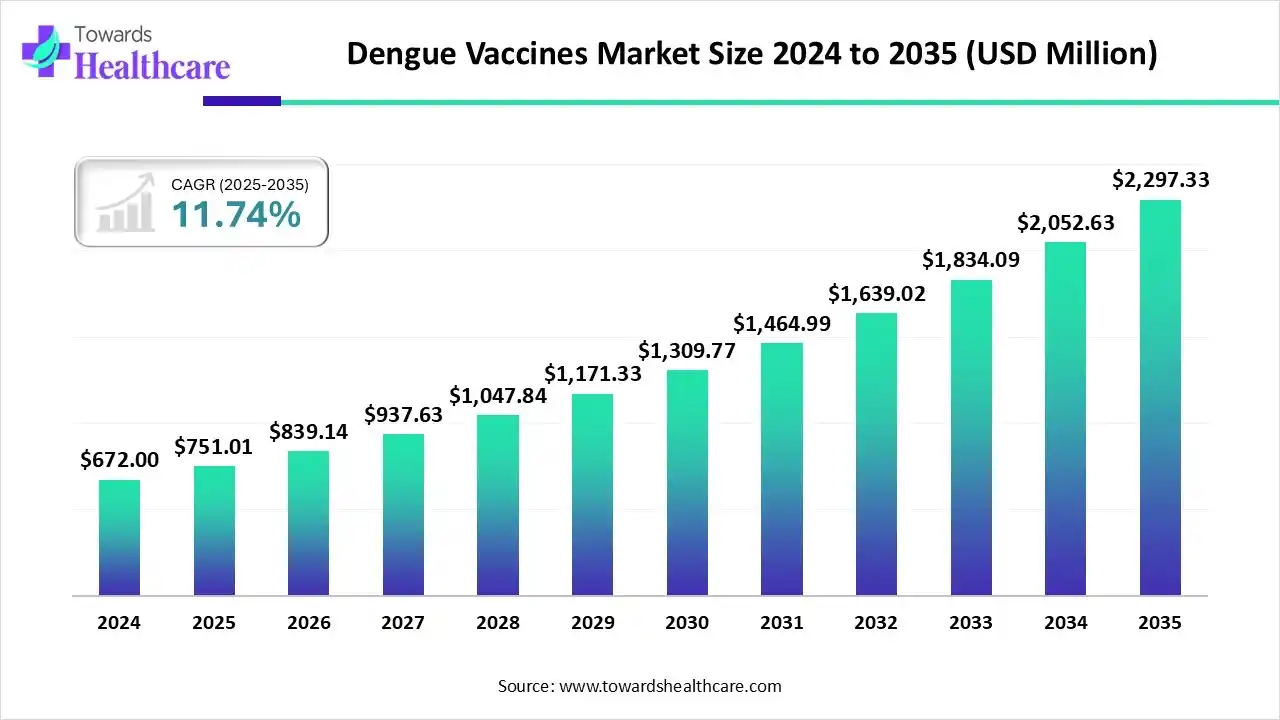
The global dengue vaccine market size is calculated at US$ 751.01 million in 2025, grew to US$ 839.14 million in 2026, and is projected to reach around US$ 2297.33 million by 2035. The market is expanding at a CAGR of 11.74% between 2026 and 2035.
Company Overview
Corporate Information
History and Background
Key Milestones/Timeline
Business Overview
A global healthcare company focused on human health through the discovery, development, manufacture, and marketing of pharmaceutical products and vaccines.
Business Segments/Divisions
Pharmaceuticals, Vaccines (Sanofi Pasteur), Consumer Healthcare.
Geographic Presence
Operates in over 70 countries worldwide, with a significant footprint in Europe, North America, and emerging markets.
Key Offerings
End-Use Industries Served
Hospitals and Specialty Clinics, Government and Public Health Agencies, Pharmacies, Retail Consumers.
Key Developments and Strategic Initiatives
Focus on strategic acquisitions to strengthen its pipeline in areas like rare diseases, immunology, and digital health.
Partnerships with academic institutions and biotechnology firms to accelerate R&D, including significant investments in mRNA technology collaborations.
Capacity Expansions/Investments
Committed to substantial annual R&D vaccine investment, approximately €1 billion.
Regulatory Approvals
Dengvaxia is approved in several endemic countries, but its use is generally restricted by the WHO to individuals with confirmed prior dengue infection due to safety concerns in seronegative individuals.
Distribution channel strategy
Utilizes a global distribution network, targeting government-led immunization programs and private healthcare channels.
Technological Capabilities/R&D Focus
Focus on next-generation vaccines, including mRNA technology platforms, to address RSV, influenza, and other infectious diseases.
Core Technologies/Patents
Holdings related to its tetravalent live attenuated dengue vaccine platform (Dengvaxia)
Research & Development Infrastructure
Global R&D centers, with a focus on advanced R&D in areas like immunology, including a commitment to make Montpellier the global hub for translational research on autoimmune diseases (October 2025).
Innovation Focus Areas
Immunology and inflammation, rare diseases, oncology, and next-generation vaccines.
Competitive Positioning
Long-standing global leader in vaccines (Sanofi Pasteur), first-to-market advantage with Dengvaxia, and broad vaccine portfolio.
Strong presence in key global vaccine markets, a critical supplier to global public health organizations.
SWOT Analysis
Recent News and Updates
Press Releases
Industry Recognitions/Awards
Continually recognized for its contributions to global health and vaccine innovation.
Company Overview
Corporate Information
History and Background
Key Milestones/Timeline
Business Overview
A patient-focused, values-based, R&D-driven global biopharmaceutical company focusing on five key business areas.
Business Segments/Divisions
Geographic Presence
Operates in over 80 countries and regions, with significant operations in Japan, the U.S., and Europe.
Key Offerings
End-Use Industries Served
Hospitals and Specialty Clinics, Government and Public Health Agencies (via immunization programs), Pharmacies.
Key Developments and Strategic Initiatives
Major acquisition of Shire plc in 2019 for approximately $62 billion, which diversified its product portfolio and global footprint.
Product Launches/Innovations
Launched Qdenga (TAK-003) as a second-generation dengue vaccine.
Capacity Expansions/Investments
Focusing on scaling up global manufacturing capacity for Qdenga, with the German facility as a primary hub and Biological E. as a key partner for future volumes and public health formats.
Regulatory Approvals
Distribution channel strategy
Strategic focus on public health programs, leveraging the WHO Prequalification for procurement by organizations like UNICEF and PAHO, alongside private market distribution.
Technological Capabilities/R&D Focus
Core technology based on a live-attenuated tetravalent vaccine, utilizing a serotype two backbone to induce robust immunity against all four serotypes (DENV-1 to DENV-4).
Core Technologies/Patents
Proprietary technology related to the DENV-2 backbone vaccine approach, Takeda's tetravalent dengue vaccine platform.
Research & Development Infrastructure
Global R&D network, including the pivotal TIDES study involving 20,000 participants across dengue-endemic countries.
Innovation Focus Areas
Complex infectious diseases, rare diseases, and advanced cell and gene therapies.
Competitive Positioning
Strengths & Differentiators
Qdenga's favorable profile for both seropositive and seronegative individuals (approved for use regardless of prior dengue exposure), strong efficacy against hospitalization, and WHO Prequalification (May 2024).
Market presence & ecosystem role
Rapidly growing presence in dengue-endemic countries, emerging as the preferred option for public health programs due to its broader label.
SWOT Analysis
Recent News and Updates
Press Releases,
Industry Recognitions/Awards
Qdenga's WHO Prequalification in May 2024 is a significant endorsement and key driver of global adoption.
To establish basic safety and immunogenicity, preclinical studies in animal models and basic research on the dengue virus are the first steps in research and development (R&D). Finding viable vaccine candidates and selecting the most promising ones for additional testing are the tasks of this stage.
Top Companies Include: Sanofi, Teva, Takeda, GSK, Merck & Co., Johnson & Johnson, Roche, Novartis, Pfizer, AstraZeneca, and Eli Lilly. Merck & Co, etc.
Phase 1 of clinical trials evaluates safety in small groups; Phase 2 studies immune response and dosage in larger groups; and Phase 3 assesses safety and efficacy in endemic areas in thousands of people. After that, data is sent to national organizations or regulatory agencies like the FDA in the form of a Biological License Application (BLA) for approval.
Top Companies Include: Sanofi, Teva, Takeda, GSK, Merck & Co., Johnson & Johnson, Roche, Novartis, Pfizer, AstraZeneca, and Eli Lilly. Merck & Co, etc.
A carefully thought-out vaccine rollout and long-term surveillance (Phase 4 trials) to track safety and effectiveness in the general population are part of patient support after approval. To guarantee proper administration, it consists of community education, precise dosage schedules (such as a three-dose series), and integration with current healthcare systems.
Top Companies Include: Merck & Co., Johnson & Johnson, Roche, Novartis, Pfizer, AstraZeneca, and Eli Lilly. Merck & Co, etc.
Collaborate with our experts to explore the Dengue Vaccine Market at sales@towardshealthcare.com