Drug-Eluting Balloon Catheters Market Leading Companies

- Abbott
- B. Braun Melsungen AG
- Bard/BD Interventional
- BIOTRONIK
- Boston Scientific
- Concept Medical
- Cook Medical
- Edward Lifesciences
- Gore & Associates
- Lépine
- MedAlliance
- Medtronic
- Philips
- Terumo Corporation
- W. L. Gore & Associates
Drug-Eluting Balloon Catheters Market Growth:
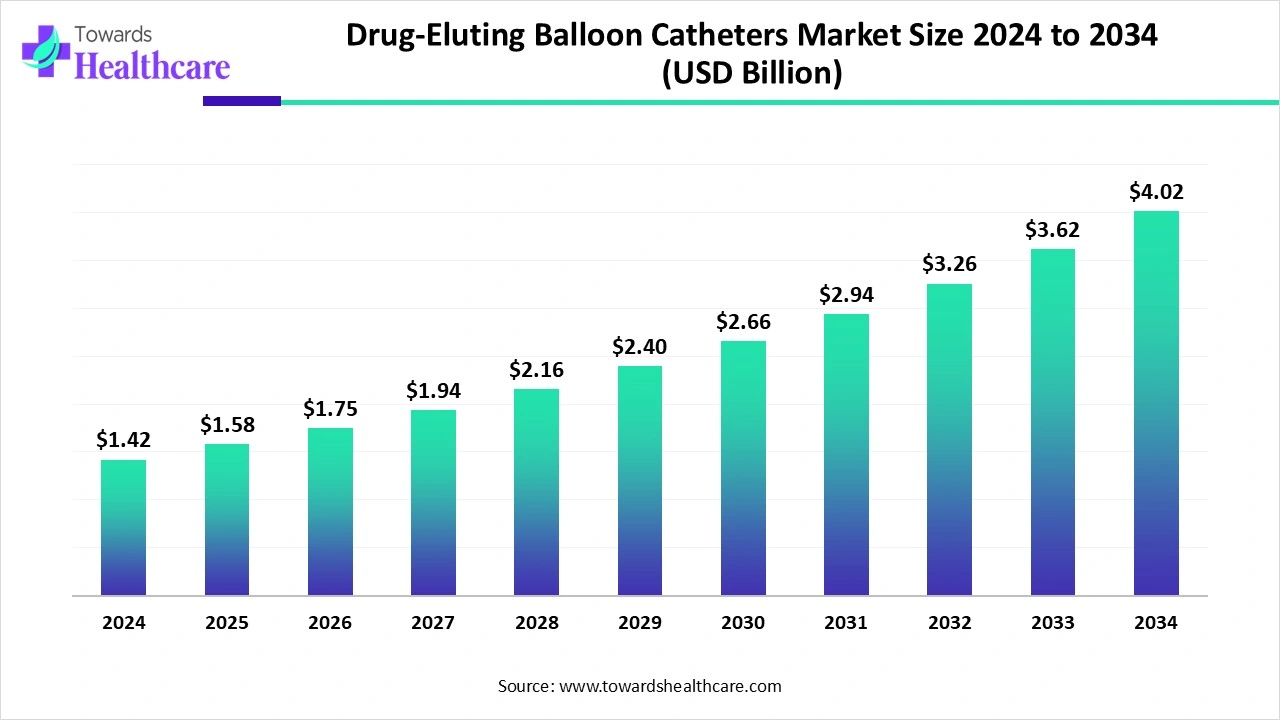
The global drug-eluting balloon catheters market size is calculated at US$ 1.42 billion in 2024, grew to US$ 1.58 billion in 2025, and is projected to reach around US$ 4.02 billion by 2034. The market is expanding at a CAGR of 11.1% between 2025 and 2034.
Trends in the Drug-Eluting Balloon Catheters Market
Numerous leaders are leveraging their position in the global market to invest in the development of novel therapies and the latest solutions for severe conditions.
- In September 2025, Concept Medical Group, a global player in drug-delivery technologies, acquired commercial approval for its flagship MagicTouch Sirolimus-Coated Balloon (SCB) for CAD treatment from ANVISA, Brazil’s Health Surveillance Agency.
- In February 2025, Teleflex Incorporated, a leading global provider of medical technologies, entered into a definitive agreement to acquire substantially all of the Vascular Intervention business of BIOTRONIK SE & Co. KG for a predicted cash payment on closing of approximately €760 million.
- In August 2023, Advanced NanoTherapies received $4M Series A extension from an undisclosed strategic investor for drug-coated balloon development.
Latest Announcements by Industry Leaders
In April 2025, Cordis, a leading player in interventional cardiovascular and endovascular technologies, announced new data from two important peripheral studies that are assessing the SELUTION SLR™ Drug-Eluting Balloon (DEB). Dr. Michael Lichtenberg, Chief of Interventional Angiology, said that this approach will deliver unequivocal clinical advantages in complex patient cohorts.
Recent Developments in the Drug-Eluting Balloon Catheters Market
- In February 2025, Cagent Vascular, Inc., the exclusive developer of serration technology for vessel dilation in endovascular interventions, launched its novel product, Serranator SL-PRO™ PTA Serration Balloon Catheter.
- In March 2024, Boston Scientific Corporation received U.S. Food and Drug Administration (FDA) approval for the AGENT™ Drug-Coated Balloon (DCB), which is used in treating coronary in-stent restenosis (ISR) in patients with coronary artery disease.
Become a valued research partner with us, please feel free to contact us at sales@towardshealthcare.com
Keypoints
- Company Overview
- Locations Subsidiaries/Geographic reach
- Key Executives
- Company Financials
- Patents registered
- SWOT Analysis
- Applications Catered
- Strategic collaborations
- Recent Developments
- Competitive Benchmarking

