Spotlight on the Companies Driving Growth in Liposomal Doxorubicin Market
- Sun Pharmaceutical Industries Ltd.
- Johnson & Johnson Services, Inc.
- Merck & Co., Inc.
- Cipla
- Shanghai Fudan-Zhangjiang Bio-Pharmaceutical Co., Ltd.
- Lupin
- Cadila Pharmaceuticals
- SRS Life Sciences
- GSK plc
- Pfizer Inc.
- Sanofi
- Sigma-Aldrich Co.
Understanding Liposomal Doxorubicin in Cancer Treatment
Liposomal doxorubicin is a chemotherapy drug in which doxorubicin is encapsulated with liposomes to enhance drug delivery, reduce toxicity, and improve cancer treatment effectiveness. The liposomal doxorubicin market is expanding due to the growing global cancer burden and increasing [reference for targeted drug delivery methods that minimize toxicity and enhance treatment efficacy.
Liposomal formulation offers improved bioavailability, prolonged circulation time, and better tumor targeting compared to conventional chemotherapy. Furthermore, continuous R&D efforts, rising approvals doe new formulations, and increasing adoption of advanced oncology treatments are contributing significantly to the market's strong growth during the forecast period.
For Instance,
- In February 2024, the National Cancer Institute estimated that global cancer cases could rise from 20 million in 2022 to 30 million by 2040, highlighting the increasing demand for advanced cancer treatment solutions.
Market Growth
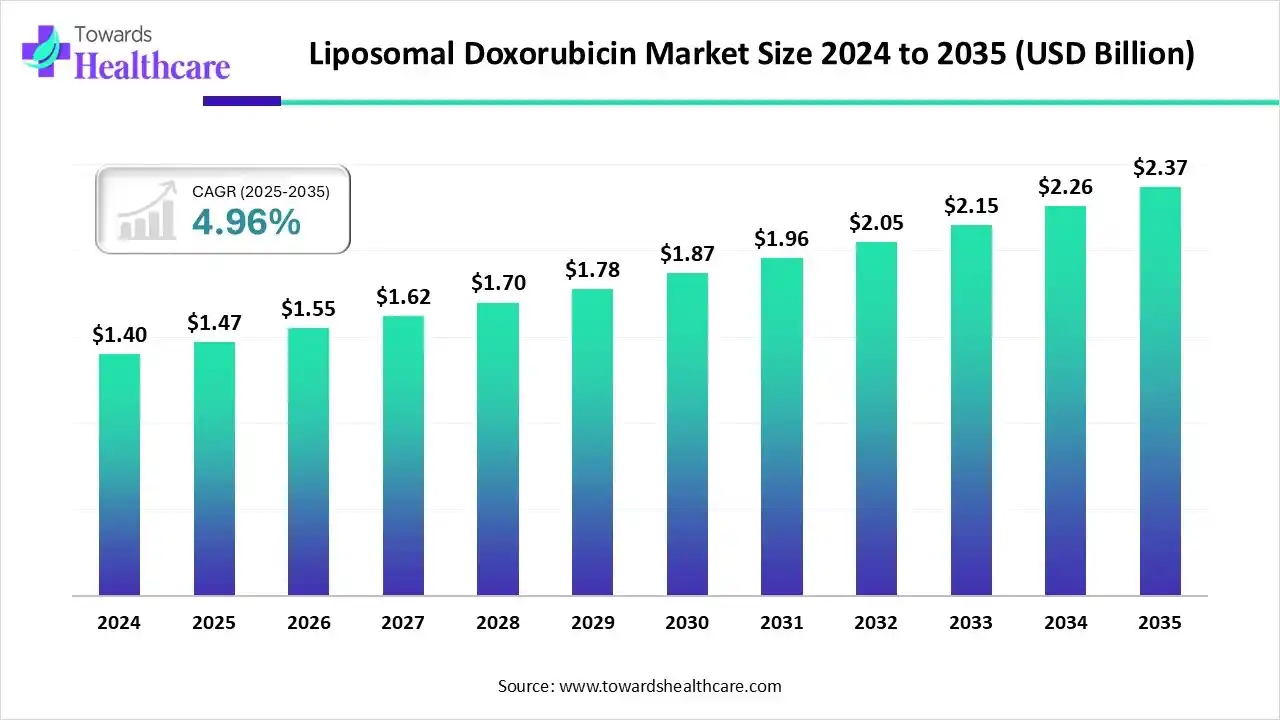
The liposomal doxorubicin market size was reported at US$ 1.47 billion in 2025 and is expected to rise to US$ 1.55 billion in 2026. According to forecasts, it will grow at a CAGR of 4.96% to reach US$ 2.37 billion by 2035.
Company And its Offerings
- Merck & Co. Inc.- Offers Keytruda (pembrolizumab) for cancer immunotherapy and is involved in advanced oncology formulation.
- Cipla Ltd- Manufactures Liposomal Doxorubicin Injection used for treating ovarian cancer, multiple myeloma, and AIDS-related Kaposi’s sarcoma.
- Lupin Limited- Launched Doxorubicin Hydrochloride Liposome Injection in the U.S. for Multiple Myeloma.
- Sanofi- Focuses on oncology biologics and rare disease therapies and is active in liposomal and targeted chemotherapy research.
- Pfizer Inc.- Known for Doxil, a leading product used to treat ovarian cancer, multiple myeloma, and breast cancer.
Company Landscape
Johnson & Johnson (Janssen Products, LP)
Company Overview
- Corporate Information, Headquarters: New Brunswick, New Jersey, US, Year Founded: 1886 (Johnson & Johnson), Ownership Type: Publicly Traded Company (NYSE: JNJ).
- History and Background, Johnson & Johnson (J&J) is one of the world's largest and most broadly-based healthcare companies. J&J acquired the rights to the original Liposomal Doxorubicin product, Doxil (marketed as Caelyx outside the US), from Sequus Pharmaceuticals.
Business Overview
- Business Segments/Divisions, Pharmaceuticals (Janssen), MedTech, Consumer Health (Recently spun off as Kenvue). The Liposomal Doxorubicin product (Doxil/Caelyx) falls under the Janssen Pharmaceuticals division, specifically within Oncology.
- Geographic Presence, Global, with a strong presence in North America and Europe, supported by its extensive international network.
- Key Offerings, The brand name, Doxil (Doxorubicin HCl Liposome Injection) in the US and Caelyx in many other regions, is a PEGylated liposomal formulation of doxorubicin.
- End-Use Industries Served, Healthcare/Pharmaceuticals, primarily focusing on Oncology (cancer treatment).
Key Developments and Strategic Initiatives
- Mergers & Acquisitions: J&J's continuous growth strategy includes strategic acquisitions to strengthen its pharmaceutical pipeline, though no recent major M&A related specifically to the Liposomal Doxorubicin product line is highlighted in 2024-2025.
- Partnerships & Collaborations: Collaborations with academic institutions and other biotech firms are ongoing across Janssen's portfolio for drug discovery and clinical trials. Janssen focuses on maintaining robust supply chain partnerships for Doxil/Caelyx manufacturing and distribution.
- Product Launches/Innovations, Focus is on maintaining the existing branded product, Doxil/Caelyx, as a proven standard of care in its indications, with ongoing research in J&J's broader oncology pipeline.
- Capacity Expansions/Investments, Significant and continuous R&D investment across the Janssen portfolio, with a focus on advancing novel oncology treatments.
- Regulatory Approvals, Doxil/Caelyx holds numerous regulatory approvals globally, including the US FDA, for its approved indications.
- Distribution channel strategy, primarily through Hospital Pharmacies and specialized distributors, often with exclusive arrangements due to the complexity and specialized nature of the cytotoxic drug.
Technological Capabilities/R&D Focus
- Core Technologies/Patents: The core technology is the proprietary PEGylated liposomal formulation, which provides prolonged circulation time and preferential accumulation at tumor sites, reducing cardiotoxicity. Although the core patents have expired, leading to generic competition.
- Research & Development Infrastructure: Extensive global R&D infrastructure under Janssen Research & Development, LLC, focusing on areas like solid tumor oncology, hematologic malignancies, and targeted therapies.
- Innovation Focus Areas, New indications, combination therapies, and post-market safety/efficacy studies for Doxil/Caelyx, while Janssen's broader focus is on novel mechanisms of action in oncology.
Competitive Positioning
- Strengths & Differentiators, First-to-market advantage and strong brand recognition (Doxil/Caelyx), proven clinical data and safety profile, established market trust among oncologists, and comprehensive global distribution network.
- Market presence & ecosystem role, Dominant market leader in branded liposomal doxorubicin, sets the benchmark for quality and efficacy in the liposomal doxorubicin segment.
SWOT Analysis
- Strengths: Established brand and trust, extensive clinical history, strong global distribution.
- Weaknesses: High cost compared to generics, original patents have expired.
- Opportunities: Exploring new combination therapies and indications, expanding market access in emerging economies.
- Threats: Aggressive competition from generic manufacturers (e.g., Sun Pharma's Lipodox), development of alternative novel oncology treatments.
Recent News and Updates
- Press Releases, Recent J&J news focuses on broader oncology pipeline approvals and advancements in new therapeutic areas, reflecting a diversified focus beyond Doxil/Caelyx.
- Industry Recognitions/Awards, J&J and Janssen consistently receive recognition for their overall R&D and pharmaceutical performance.
Sun Pharmaceutical Industries Ltd.
Company Overview
- Corporate Information, Headquarters: Mumbai, Maharashtra, India, Year Founded: 1983, Ownership Type: Publicly Traded Company (NSE: SUNPHARMA, BSE: 524715).
- History and Background, Sun Pharma is a leading global specialty generic company, the largest pharmaceutical company in India, and a key player in specialty generics globally. Its Liposomal Doxorubicin product, Lipodox, gained significant market share, particularly in the US and emerging markets, often during periods of Doxil/Caelyx shortages.
Business Overview
- Business Segments/Divisions, Specialty Generics, Generics, Active Pharmaceutical Ingredients (API), and Consumer Healthcare. Lipodox is a key product in its Oncology portfolio under its Specialty Generics and Global Generics divisions.
- Geographic Presence, Global footprint in over 100 countries, with major markets including India (No. 1 pharma company), the US (significant generic presence), Europe, and emerging markets.
- Key Offerings, Lipodox (Doxorubicin Hydrochloride Liposome Injection), a generic equivalent of Doxil/Caelyx, available in various strengths.
- End-Use Industries Served, Healthcare/Pharmaceuticals, primarily serving the Oncology market with a focus on accessible and affordable cancer treatments.
Key Developments and Strategic Initiatives
- Mergers & Acquisitions, Sun Pharma continuously engages in strategic acquisitions to bolster its generics and specialty pipeline, such as its past acquisition of Ranbaxy.
- Partnerships & Collaborations, Strategic alliances and partnerships are a core part of its business model to expand its global reach and access to complex generics.
- Product Launches/Innovations, Continuous focus on launching complex generics and specialty products, as seen by the US FDA approval and launch of their generic Liposomal Doxorubicin product. In August 2024, Lupin Limited (a competitor, but highlighting generic market activity) launched a generic Doxorubicin Hydrochloride Liposome Injection in the US, increasing the competitive pressure in the generic segment. Sun Pharma’s focus is on complex generics, including liposomal drugs.
- Capacity Expansions/Investments, Substantial R&D investments (6-8% of global revenues) focused on formulations, process chemistry, and complex generics like liposomal drugs and inhalers. Expanding manufacturing capacity across its 40+ global facilities.
- Regulatory Approvals, Holds US FDA approval for its generic Doxorubicin Hydrochloride Liposome Injection (Lipodox), along with approvals in multiple international markets.
- Distribution channel strategy, Leverages its robust generics distribution network to target both hospital pharmacies and retail/online pharmacies globally, prioritizing cost-effectiveness and broad availability.
Technological Capabilities/R&D Focus
- Core Technologies/Patents, Expertise in complex generics, including specialized formulations like liposomes (nanotechnology) for improved drug delivery. Possesses strong capabilities in API and formulation development.
- Research & Development Infrastructure, Extensive R&D team (over 2,900 personnel) with a focus on differentiated products, including liposomal injections, inhalers, and controlled-release dosage forms.
- Innovation Focus Areas, Developing next-generation complex generics, innovative specialty medicines in areas like oncology, and enhancing drug delivery systems to improve patient outcomes and address unmet needs.
Competitive Positioning
- Strengths & Differentiators, Cost-effective alternative to branded products, strong presence in emerging markets, deep expertise in complex generic formulations (Liposomal technology), robust global manufacturing and supply chain.
- Market presence & ecosystem role, Leading player in the generic segment of the Liposomal Doxorubicin market, acting as a crucial provider of affordable cancer treatments, and a major player in the global generics ecosystem.
SWOT Analysis
- Strengths: Lower price point driving market penetration, strong global generic supply chain, focus on complex generics technology.
- Weaknesses: Faces competition from multiple generic players, perceived as a generic, not an innovator brand.
- Opportunities: Expanding presence in regulated markets (US, Europe), leveraging strong API capabilities, and increasing global demand for affordable cancer drugs.
- Threats: Price erosion due to increasing generic competition, stringent regulatory scrutiny in developed markets, and dependence on generic market dynamics.
Recent News and Updates
- Press Releases, Recent news highlights overall strong financial performance, expansion in specialty businesses, and consistent focus on complex product approvals.
- Industry Recognitions/Awards, Frequently recognized as a top pharmaceutical company in India and a significant global generics player.
Liposomal Doxorubicin Market Value Chain Analysis
Clinical Trials
- (PLD), marketed as Doxil or Caelyx, offers a safer and more targeted alternative to conventional doxorubicin.
- Its liposomal formulation minimizes cardiotoxicity and other adverse effects.
- Clinical studies confirm its strong efficacy in treating various cancer types with improved patient safety.
Packaging and Serialization
- Liposomal doxorubicin products like Doxil require highly regulated packaging and serialization standards.
- The packaging safeguards the stability and integrity of liposomes against temperature fluctuations and contamination.
- Serialization provides full traceability, ensuring product authenticity and preventing counterfeiting across the supply chain.
Patient Support and Services
- Patient support for liposomal doxorubicin includes collaboration with healthcare providers and cancer care organizations.
- Services offer clinical guidance, financial aid, and emotional support tailored to patient needs.
- The type and availability of support vary based on cancer type, manufacturer programs, and regional access.
Recent Developments in the Liposomal Doxorubicin Market
- In June 2025, Alembic Pharmaceuticals Limited received USFDA approval for its Doxorubicin Hydrochloride Liposome Injection, expanding its oncology portfolio. The drug is approved for treating multiple myeloma, ovarian cancer, and AIDS-related Kaposi Sarcoma, enhancing access to advanced cancer therapies in the U.S. market.
- In January 2024, CHEPLAPHARM Group acquired the commercial rights for Myocet from Teva in Europe, marking its first product purchase from the company. This move expanded CHEPLAPHARM’s oncology portfolio, with Myocet being used as a first-line treatment for metastatic breast cancer in adult women.
Case Study: Alembic Pharmaceuticals’ Expansion in Oncology with USFDA Approval for Doxorubicin Hydrochloride Liposome Injection
Overview
In June 2025, Alembic Pharmaceuticals Limited achieved a significant milestone by receiving approval from the U.S. Food and Drug Administration (USFDA) for its Doxorubicin Hydrochloride Liposome Injection. This approval marks a major step in Alembic’s expansion within the oncology segment, reinforcing its commitment to offering advanced cancer treatment options. The injection is approved for the treatment of multiple myeloma, ovarian cancer, and AIDS-related Kaposi Sarcoma, positioning Alembic as a key contributor to improving access to essential oncology drugs in the U.S. market.
About the Case Study
This case study highlights Alembic’s strategic approach to strengthening its oncology portfolio through innovation and regulatory excellence. By introducing a liposomal formulation of Doxorubicin Hydrochloride, a well-established chemotherapy drug, Alembic enhances drug delivery efficiency while minimizing side effects. The company’s USFDA approval not only validates its research and manufacturing capabilities but also underscores India’s growing influence in global pharmaceutical innovation.
Challenges Faced
-
Regulatory Complexity: Securing USFDA approval involves stringent testing, documentation, and manufacturing standards that require robust compliance systems.
-
Formulation Difficulty: Developing a liposomal drug formulation demands precision in encapsulation technology to ensure controlled drug release and stability.
-
Market Competition: The oncology drug segment in the U.S. is highly competitive, dominated by established global pharmaceutical giants.
-
Clinical Validation: Demonstrating the efficacy and safety of the liposomal formulation compared to traditional versions required extensive clinical evaluation.
Solutions Implemented
-
Investment in Advanced R&D: Alembic invested in state-of-the-art R&D infrastructure and collaborated with formulation scientists to develop a high-quality liposomal product.
-
Regulatory Strategy: The company implemented a strong regulatory framework, ensuring compliance with USFDA requirements for both product quality and manufacturing practices.
-
Technology Adoption: Advanced liposome encapsulation technology was utilized to improve drug bioavailability and reduce toxicity.
-
Strategic Focus on Oncology: Alembic’s oncology-focused product strategy ensured consistent portfolio growth and alignment with unmet patient needs.
Key Results or Outcomes
-
USFDA Approval: Official recognition of Alembic’s formulation quality and compliance with international standards.
-
Expanded Oncology Portfolio: Strengthening Alembic’s position in the high-growth oncology market segment.
-
Improved Patient Access: The approval allows U.S. patients broader access to advanced, cost-effective cancer therapies.
-
Global Market Recognition: The milestone reinforces Alembic’s reputation as a global player capable of delivering innovative treatments.
Steps or Strategies Taken
-
Conducted in-depth research on liposomal technology and its application in oncology drugs.
-
Ensured adherence to international Good Manufacturing Practices (GMP).
-
Established a specialized oncology division to focus on product development and clinical testing.
-
Engaged with regulatory authorities early in the approval process to streamline submissions and compliance.
-
Developed a market-entry plan targeting U.S. oncology healthcare providers and distributors.
Conclusion
Alembic Pharmaceuticals’ USFDA approval for Doxorubicin Hydrochloride Liposome Injection represents more than just a product launch, it showcases the company’s strategic shift towards innovation-driven healthcare. By combining cutting-edge formulation technology with regulatory excellence, Alembic not only expands its oncology footprint but also contributes to global cancer care accessibility. This milestone underscores how Indian pharmaceutical firms are increasingly playing a pivotal role in shaping the future of advanced therapeutics worldwide.
Partner with our experts to explore the Liposomal Doxorubicin Market at sales@towardshealthcare.com

