January 2026

The global cell-based immunotherapies market size is calculated at USD 4.59 billion in 2024, grow to USD 5.28 billion in 2025, and is projected to reach around USD 18.62 billion by 2034. The market is expanding at a CAGR of 15.02% between 2025 and 2034.
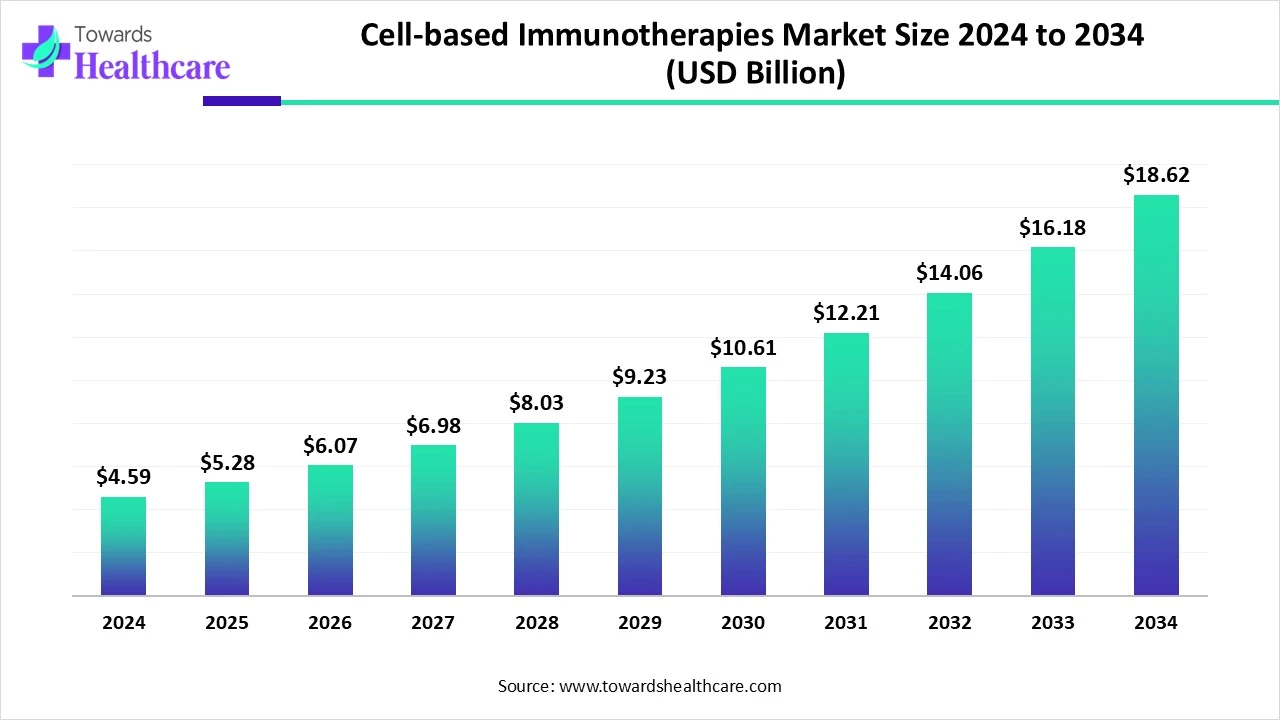
The cell-based immunotherapies market is witnessing strong growth due to the rising burden of cancer and advancements in immunology and cell engineering. Therapies like CAR T-cell and TCR-based treatments are gaining traction for their targeted approach and lasting effectiveness. Growing clinical research, regulatory support, and increasing demand for personalized therapies are further propelling the market forward, especially as traditional treatments face limitations in complex or resistant cancers.
| Metric | Details |
| Market Size in 2025 | USD 5.28 Billion |
| Projected Market Size in 2034 | USD 18.62 Billion |
| CAGR (2025 - 2034) | 15.02% |
| Leading Region | North America share by 45.50% |
| Market Segmentation | By Therapy Type, By Application/Indication, By Source of Cells, By End Use, By Regions |
| Top Key Players | Novartis AG, Gilead Sciences (Kite Pharma), Bristol-Myers Squibb (Juno Therapeutics), Adaptimmune Therapeutics, Fate Therapeutics, bluebird bio, Inc., Allogene Therapeutics, Celyad Oncology, TCR2 Therapeutics, Immunocore Limited, Bellicum Pharmaceuticals, Atara Biotherapeutics, Cellular Biomedicine Group, Medigene AG, Legend Biotech, Takeda Pharmaceutical Company, Mustang Bio, Precision BioSciences, Iovance Biotherapeutics, Celyad Oncology |
Cell-based immunotherapies refer to advanced therapeutic approaches that use living cells to treat diseases by harnessing or modifying the immune system's natural ability to fight illnesses, particularly cancers, autoimmune disorders, and infectious diseases. These therapies include genetically engineered or naturally derived immune cells designed to enhance the body’s immune response, such as T cells, dendritic cells, and natural killer cells.
The market includes research, development, manufacturing, and commercialization of these therapies for clinical use. Innovation is transforming the cell-based immunotherapies market by enhancing treatment precision, safety, and effectiveness. Breakthroughs in gene editing, synthetic biology, and cell manufacturing have led to next-generation therapies like CAR-T cells. With reduced side effects and broader applications. These advancements are accelerating clinical success and expanding the use of immunotherapies beyond oncology into autoimmune and infectious diseases.
AI is significantly influencing the market by accelerating drug discovery, optimizing clinical trial design, and enhancing patient selection through predictive analytics. It helps identify precise cellular targets, improves genetic modification techniques, and streamlines manufacturing processes. By analyzing large datasets, AI supports personalized treatment planning and early detection of therapy responses. These advancements reduce development time and costs, making innovative immunotherapies more accessible and effective across a wider range of conditions.
Rising Prevalence of Cancer
The global search for cancer cases is fueling the growth of the cell-based immunotherapies market, as there is a growing need for more precise and long-lasting treatments. Unlike conventional methods, these therapies provide targeted action with fewer side effects by modifying immune cells to attack cancer specifically. This increased demand for safer and more personalized treatments encourages research, development, and adoption of cell-based immunotherapies, positioning them as a critical solution in modern oncology care.
High Treatment Cost and Manufacturing Complexity
The cell-based immunotherapies market faces restraints due to the intensive resources needed for production and delivery. These therapies demand sophisticated labs, cold chin logistics, and specialized handling, all contributing to elevated costs. As, result, affordability becomes a major concern for both patients and healthcare providers. Limited reimbursement options and access issues, especially in developing regions, further restrict widespread adoption making high cost and complexity a significant hurdle to market expansion.
Expansion into the Cell-based Immunotherapies Market
The treatment of solid tumors represents a major growth avenue for cell-based immunotherapies. While current therapies are largely limited to blood cancers, emerging approaches like TCR-T and TIL therapies are showing potential in targeting solid tumor cells effectively. With advancements in tumor microenvironment modulation and antigen targeting, these therapies could be adapted for a wider range of cancers. This untapped segment offers a vast patient base and is expected to significantly boost market expansion.
In 2024, CAR-T therapy held the largest revenue share in the market due to its rapid clinical adoption and strong therapeutic outcomes in targeting blood cancer. Its personalized approach, where a patient’s T-cells are modified to target cancer cells, showed remarkable remission rates. Growing approvals, increased investment in CAR-T R&D, and expanding application beyond hematologic malignancies helped drive its dominance, positioning it as a leading therapy type in the evolving immunotherapy landscape.
For Instance,
The NK cell therapy segment is anticipated to witness the fastest growth due to its unique ability to destroy tumor cells without prior antigen exposure. Unlike T-cell therapies, NK cells pose a lower risk of graft-versus-host disease, making them suitable for off-the-shelf use. Advancements in CAR-NK technology, improved safety profiles, and expanding clinical trials across multiple cancers are driving interest. These advantages position NK cell therapies as a promising next-generation option in cell-based cancer treatment.
In 2024, the oncology segment dominated the cell-based immunotherapies market owning to the rising demand for targeted cancer treatment and growing success of immune-based therapies. The increasing incidence of various cancers and favorable outcomes from therapies like CAR-T accelerated adoption. Strong research funding and clinical advancements further reinforced oncology leading market position.
In 2024, hematologic malignancies held the top revenue share in the cell-based immunotherapies market due to the early and widespread clinical success of therapies like CAR-T in blood-related cancers. These cancers are often more accessible to immune cells, Allowing better treatment outcomes. Continued innovation and strong clinical backing further boosted the market growth.
The solid tumor segment is expected to grow at the fastest CAGR due to rising efforts to overcome past challenges in treating these cancers with cell-based therapies. Breakthroughs in engineering immune cells to better penetrate tumor environments and target specific antigens have shown encouraging early results. As Solid tumors account for the majority of cancer cases, expanding research and clinical trials are fueling interest and investment, driving rapid growth in the application area.
The autologous segment led the cell-based immunotherapies market in 2024 due to its high safety profile and strong clinical outcomes. Using a patient’s cells reduces complications like graft-versus-host disease and improves compatibility. This approach is widely adopted in therapies such as CAR-T for blood cancers. Growing interest in customized treatment and fewer regulatory hurdles also supported the market dominance position in the market.
The allogeneic segment is set to grow rapidly as it enables the development of off-the-shelf therapies that can treat multiple patients from a single donor source. This improves accessibility and reduces treatment delays. Innovations in immunomodulation and reduced risk of rejection are making these therapies more viable. Their potential for large-scale production is drawing strong interest from developers and investors, supporting accelerated growth during the forecast period.
In 2024, the hospital and clinics segment dominated the cell-based immunotherapies market because of their ability to manage complex procedures and critical care needs. These settings are equipped with advanced medical technologies and trained staff necessary for handling personalized cell therapies. Their integration with diagnostic and follow-up services ensures better patient outcomes, making them the primary choice for delivering such treatment and contributing to higher revenue generation.
The specialty cancer centers segment is projected to grow rapidly as these facilities are increasingly becoming preferred sites for advanced immunotherapy procedures. Their dedicated focus on cancer care allows for faster integration of novel treatments, supported by expert oncologists and tailored care models. With rising referrals for complex therapies and growing participation in research, these centers are positioned to lead future advancements in cell-based immunotherapy delivery.
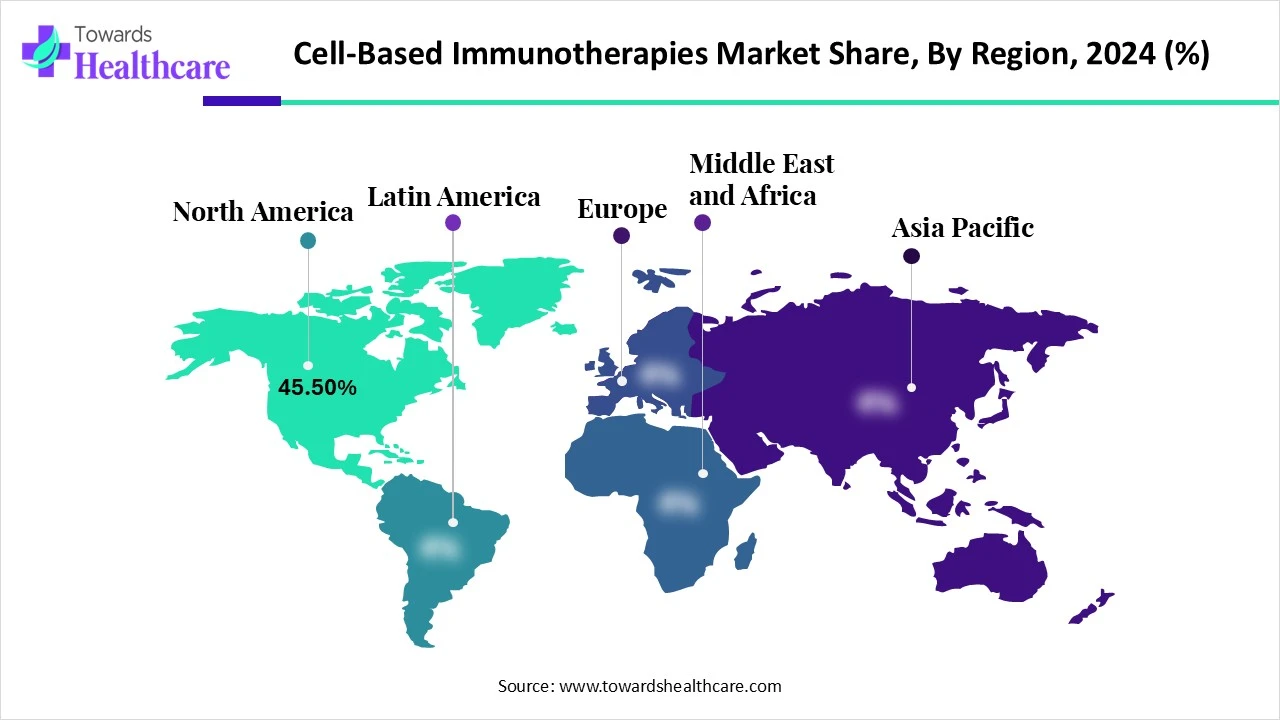
North America leads the cell-based immunotherapiesmarket share by 45.50% in 2024 owing to its well-established biotech ecosystem, growing focus on personalized medicine, and robust funding for cancer research. The region benefits from a high number of clinical trials, skilled professionals, and early regulatory approvals for novel therapies. Moreover, strong collaboration between academic institutions and industry players accelerates innovation, making North America a key hub for the advancement and commercialization of cell-based immunotherapeutic solutions.
The U.S. market is growing due to a surge in cancer incidence, rising demand for targeted treatments, and rapid advancements in biotechnology. Government support through research grants, along with strong private sector investment, fuels innovation and development. Additionally, the presence of major pharmaceutical companies, a favorable regulatory environment, and increased patient awareness of personalized therapies contribute to the expansion of this market across various therapeutic areas in the country.
Canada's market is expanding due to the rising incidence of cancer and chronic diseases has heightened the demand for advanced treatments. Government initiatives and funding are bolstering research and development in this sector. Additionally, Canada's robust healthcare infrastructure and increasing collaborations between academic institutions and biotech firms are accelerating clinical trials and the adoption of innovative therapies. These elements collectively contribute to the market's significant growth trajectory.
Asia-Pacific is anticipated to witness the fastest CAGR in the market due to rising healthcare investments, expanding biotech infrastructure, and growing awareness of precision medicine. Increasing clinical trial activity, supportive government initiatives, and a surge in regional collaborations with global pharma companies are accelerating innovation. Moreover, the high burden of cancer and improved access to advanced treatments are encouraging adoption, making the region a promising growth frontier in this field.
China's market is expanding rapidly due to a combination of factors, including the government has implementation of supportive policies and expedited regulatory approvals, fostering a conducive environment for innovation. Significant investments in research and development have led to a surge in clinical trials, particularly in CAR-T therapies. Additionally, the country's large patient population provides a substantial base for clinical studies and therapy adoption. These elements collectively contribute to the robust growth of China's cell-based immunotherapy sector.
India’s market is growing due to increasing local innovations, such as the development of indigenous CAR-T therapies. The rise in oncology cases, along with improving access to advanced diagnostics and treatment, is fueling demand. Strong academic-industry partnerships, expanding biotech startups, and growing clinical research capabilities further support market expansion. Additionally, cost-effective manufacturing and rising healthcare awareness are making these therapies more accessible across the country.
In 2024, Europe is advancing its market through increased R&D investments, supportive regulatory frameworks, and strategic collaborations. The European Union's funding initiatives, such as Horizon Europe, are bolstering research in personalized medicine and advanced therapies. Countries like Germany, France, and the UK are leading in clinical trials and approvals, with innovations in CAR-T cell therapies and tumor-infiltrating lymphocytes. Additionally, partnerships between academia and industry are accelerating the development and commercialization of novel immunotherapies across the region.
The UK's market is expanding due to increased investment in research and development, a surge in early-phase clinical trials, and the establishment of new manufacturing facilities. In 2024, the number of Phase I advanced therapy clinical trials in the UK grew by approximately 70%, indicating a robust pipeline of innovative therapies. Additionally, the UK government has committed £520 million to life sciences manufacturing between 2025 and 2030, further bolstering the sector's growth. These factors collectively contribute to the UK's strong position in the cell-based immunotherapy market.
Germany’s market is witnessing growth due to the country's focus on cutting-edge medical innovations and expanding biotech collaborations. The presence of key pharmaceutical players, along with increased funding for personalized cancer therapies, is driving momentum. Furthermore, Germany's emphasis on translational research, academic excellence, and rising clinical success rates in treating various cancers is boosting confidence and accelerating the adoption of advanced immunotherapy solutions across the healthcare system.

In May 2024, Researchers at The Ohio State University Comprehensive Cancer Center (OSUCCC – James) reported encouraging results from a global phase II clinical trial evaluating lifileucel, a tumor-infiltrating lymphocyte (TIL) therapy, for stage 4 non-small cell lung cancer. Published in Cancer Discovery, the study showed that lifileucel, which uses a patient’s lymphocytes to attack cancer, was both safe and effective in patients who had developed resistance to immune checkpoint inhibitors. Dr. Kai He emphasized its potential as a much-needed treatment option for advanced lung cancer cases.
By Therapy Type
By Application/Indication
By Source of Cells
By End Use
By Region
January 2026
January 2026
January 2026
January 2026