December 2025

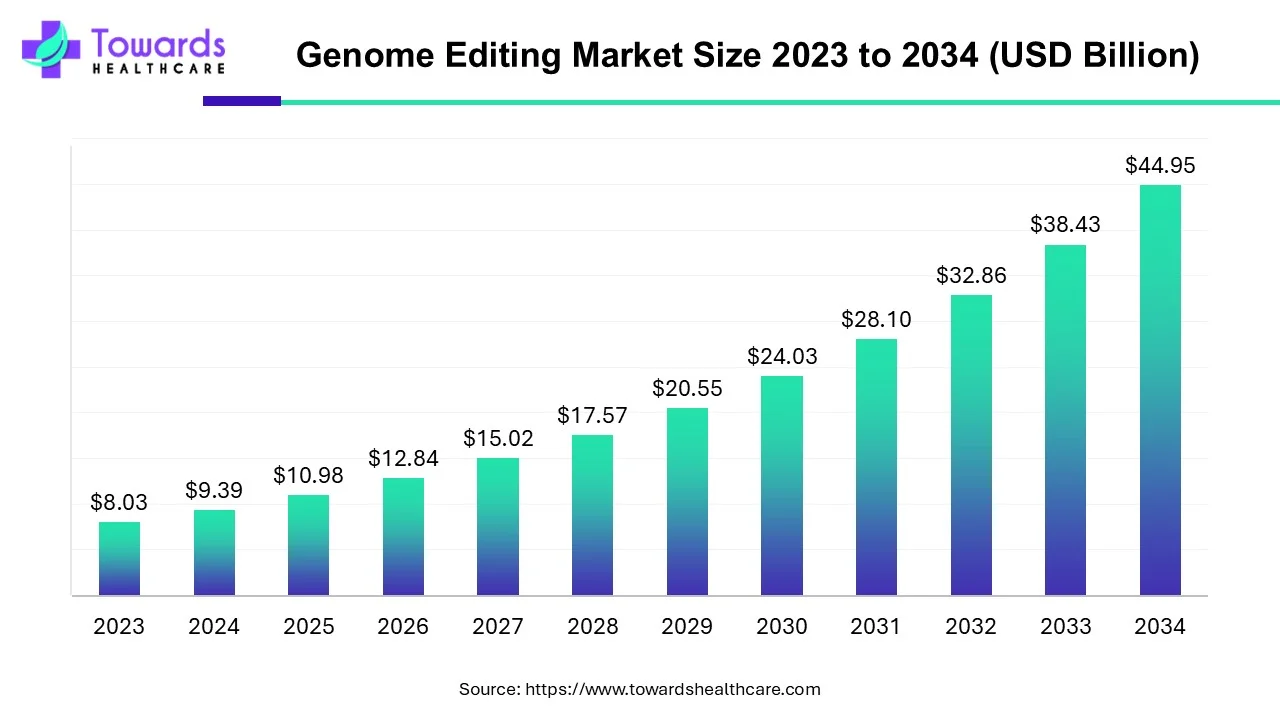
The genome editing market is expected to grow from USD 10.98 billion in 2025 to USD 44.95 billion by 2034, with a CAGR of 16.95% throughout the forecast period from 2025 to 2034.
The genome editing market offers a range of techniques for changing the genetic makeup of living organisms, is booming, and represents the cutting edge of biotechnological innovation. Thanks to a suite of technologies known as genome editing, also sometimes called gene editing.
Scientists may now modify an organism's DNA. With the use of these techniques, genetic material may be added, removed, or modified at certain genomics locations. Many techniques have been developed to alter DNA. Unlike previous genetic engineering techniques that randomly inserted genetic material across the genome, genome editing targets specific locations inside the host genome for genetic material insertions.
The genome editing market is significantly growing due to its application in research, disease diagnosis & treatment, genetically modified organizations, drug discovery, therapeutics development, and so on. Technological advancements and continuous innovation by key organizations and governments will boost the growth of the market in the future.
The AI models are integrated into medical care systems to assist clinicians in making up-to-date decisions about gene editing. These models provide insights into potential genetic conditions, detect optimum gene editing approaches, and predict patient responses to particular interventions. AI-driven promises in enhancing numerous facets of the genome editing method, which causes the growth of genome editing market.
For instance,
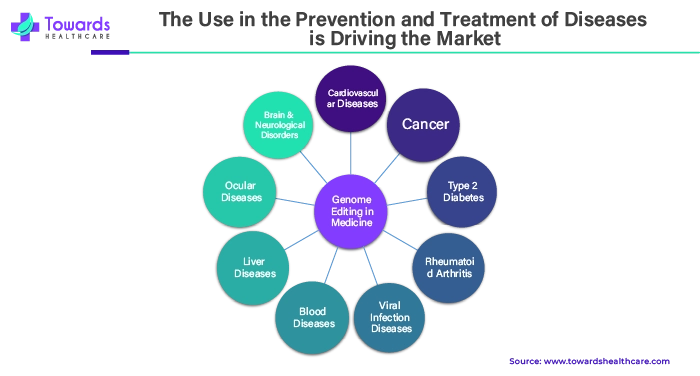
With further use in the works, genome editing is already or will soon be available in clinics to treat a number of disorders that are driving the growth of the genome editing market. Active measures must be taken to guarantee that this ground-breaking technology is used responsibly to treat, cure, and prevent genetic illnesses, given the field's fast speed.
With the advent of high-throughput genomic screening, comparative analysis of human genome sequences, and lower costs associated with genome sequencing, the field of genetic illness diagnosis has advanced quickly. The possibility of correcting disease-causing germline mutations by genome editing presents a way to induce genetic alterations that would be inherited by subsequent generations.
For instance,
Although CRISPR has a great deal of potential as a genome-editing tool, there are still a few issues that need to be resolved before applying. One of the biggest obstacles to CRISPR gene editing is off-target DNA cleavage, which happens when the molecular scissors cut into the wrong part of the host genome, changing the genome in a way that is non-specific, undesirable, unanticipated, or even harmful, rather than correcting these flaws. The mechanism creates genetic mutations. Many techniques, including prime editing, off-target identification, sgRNA optimization, Cas9 nuclease modification, and others, have been developed to lessen the impacts of off-targeting.
Millions of people worldwide suffer from rare genetic disorders. The majority of them are brought on by faulty genes that lower life expectancy and increase the risk of dying young. Genetic therapies are seen to be the most promising treatment for uncommon genetic illnesses since they attempt to correct or replace damaged genes. Currently, only around 5 percent of rare genetic illnesses have approved medicines. These diseases have few therapeutic alternatives. However, it is anticipated that genetic medicines will transform the management of uncommon genetic illnesses in the future.
By application, the genetic engineering segment dominated the market. Genome editing is a type of genetic engineering. It is a crucial research tool that makes it possible to examine the role of particular genes. Medications, vaccines, and other goods have been extracted from creatures that were genetically modified to generate them. Crops have been designed to increase production, nutritional value, and resistance to environmental pressures in order to help ensure food security. For natural scientists, genetic engineering is a valuable tool, and the development of transgenic organisms is one of the most significant tools for the study of gene function.
For instance,
By application, the clinical application segment is expected to grow at the fastest rate during the forecast period. Gene therapy, medication production, and the development of model animals with characteristics similar to human health are just a few of the numerous medical uses of genetic engineering. Human albumin, monoclonal antibodies, antihemophilic factors, follicle-stimulating hormones (used to treat infertility), human growth hormones, and several other medications have all been subject to this use. Genome editing is becoming increasingly necessary because of the increasing number of genetic abnormalities brought on by population growth and changing environmental factors.
For instance,
By technology, the CRISPR/Cas9 segment dominated the market and is expected to grow at the fastest CAGR during the forecast period. Because of its straightforward operation, simple design, low cost, and great efficiency, CRISPR/Cas9 technology has emerged as the most studied gene editing technique in recent years. Studying genes or genomic functions in humans, animals, plants, and microbes has demonstrated significant promise thanks to CRISPR/Cas9. Technology utilizing CRISPR/Cas9 has the ability to not only treat diseases but even modify aging and extend human lifespans. These arguments support the idea that further research on the CRISPR/Cas system's potential applications will be beneficial to humanity in the long run. It is possible to preserve physical health conditions and treat all incurable diseases by using the CRISPR/Cas system.
For instance,
By technology, the ZFN is expected to grow with significant growth during the forecast period. Targetable DNA cleavage reagents known as zinc-finger nucleases (ZFNs) have been found to be widely used as instruments for targeting certain genes. ZFNs are a revolutionary tool with the potential to change personalized therapy and improve biological research. Numerous plants and animals can have their genomes altered with the use of zinc finger nucleases. ZFNs are also employed in the development of isogenic human illness models, a new class of genetic disease models. All of the issues related to the viral delivery of therapeutic genes may be avoided with the plasmid-based strategy that encodes ZFN.
By the delivery method, the ex-vivo segment dominated the market. In this delivery method, cells are removed from the body, genetic material is altered, and then the modified cells are delivered back into the body. One benefit of the ex vivo delivery method is that the entire process of generating new genetic material may be managed. It is possible to confirm the new genetic material's health using ex vivo techniques prior to a person receiving it. Blood-related illnesses are the most common uses of ex vivo gene therapy.
This covers various malignancies, including certain forms of lymphoma, leukemia, and hereditary illnesses. Future uses for ex vivo gene therapy could include treating diseases other than blood and neurological conditions brought on by a genetic flaw. Researchers are trying to find treatments for autoimmune illnesses like lupus, neurological disorders like leukodystrophies, and other hereditary abnormalities. They are also trying to cure a compromised, malfunctioning, or aberrant immune system, boosting this segment's growth in the genome editing market.
By the delivery method, the in-vivo segment is estimated to grow at the fastest rate during the forecast period. In vivo, gene therapy allows for natural cell growth by directly inserting newly generated genetic material, typically DNA, into the cells. For genetic illnesses affecting a single gene in the body, in vivo approaches could be most advantageous. Additionally, targeting an inside organ like the heart is believed to be more advantageous. Targeting internal organs is made simpler by in vivo techniques. Furthermore, since removing a person's cells is unnecessary, it is less complex.
By end-use, the biotechnology and pharmaceutical companies dominated the market. In biotechnology, genome editing is a field that has long been researched and exploited. Genome editing is used in biotechnology to investigate the roles of various genes. In addition, it is employed in the development of genetically modified organisms for a range of applications, including the production of vaccines, the development of genetically modified crops, the development of plant- or microbe-based products, such as human insulin, and so on. Vaccines and monoclonal antibodies are only two examples of the biopharmaceuticals that are now made using genetic engineering methods. By modifying the host organisms for increased yields and purity, genetic engineering enables researchers to maximize the synthesis of medicinal substances. The creation of tailored medicines is made possible by genetic engineering, which is crucial in this regard.
By region, North America dominated the genome editing market in 2023. North America is known for its technological advancements, research, and development, which have led the market in the North American region. Countries like the U.S. and Canada significantly contribute to the market's growth. The majority of GMOs are regulated by three federal entities that are part of the US government. The term "genetically modified organism," or "GMO," is now often used by the public and media to refer to any plant, animal, or microorganism whose genetic material (DNA) has been modified by a procedure known as genetic engineering. The safety of genetically modified organisms (GMOs) for human, plant, and animal health is guaranteed by the U.S. Department of Agriculture (USDA), the Environmental Protection Agency (EPA), and the Food and Drug Administration (FDA).
U.S. Market Trends
About 30 million people in the U.S. are affected by rare diseases in the U.S. The U.S. government supports the use of genomic technologies for the development of genome products for the prevention, diagnosis, and treatment of genetic disorders. In May 2025, the Children’s Hospital of Philadelphia treated the world’s first patient with personalized CRISPR Gene Editing Therapy.
Canada Market Trends
It is estimated that around 1 in 12 Canadians are affected by rare diseases, accounting for 2.5 million people. The Canadian government recently launched the Canadian Genomics Strategy with an investment of $175.1 million over 7 years starting from 2024-25. The strategy was launched to translate genomic research into real-world applications.
By region, Asia Pacific is expected to grow at the fastest rate during the forecast period. Asia Pacific is the largest continent and is significantly contributing to the growth of the genome editing market. The market is growing due to countries like China, Japan, India, and South Korea. These countries are showcasing technological advances and government investment for the growth of biotechnology, genetic engineering, agriculture, and healthcare.
India Market Trends
The Department of Biotechnology (DBT) of the Government of India is spearheading the push to promote biotechnological innovation and entrepreneurship by harnessing the potential of strategic partnerships and building capacity across the country. From 6 in 2014 to over 75 in 2023, the number of bio-incubators increased. Approximately INR 4,500 Cr has been raised in follow-on financing by 150 enterprises. The bioeconomy of the country was estimated to be worth $137 billion in 2023 and is expected to reach $300 billion by 2030.
China Market Trends
China is home to 544 genomics startups, of which 318 are funded. The Chinese Ministry of Science and Technology recently published ethical guidelines to regulate human genome editing research and promote its development. This supports healthcare professionals to evaluate and address the severity of the disease and potential risks.
Europe is expected to grow at a considerable CAGR in the genome editing market in the upcoming period. The rising prevalence of genetic and rare disorders and the rising adoption of advanced technologies augment the market. Government organizations support genome editing through funding. They also create awareness among the general public to screen for and diagnose genetic disorders. The increasing investments and collaborations among key players facilitate market growth. The burgeoning genomic and molecular diagnostics sector contributes to market growth.
UK Market Trends
In July 2025, the UK government announced a 10-year health plan for England to revolutionize the role of genomics in the healthcare sector. The NHS Genomics Medicine Service will expand population health testing and shorten testing times. The Genomics England-led Generation Study recruits up to 100,000 newborn babies and screens them for over 200 genetic conditions.
| GAs (N=10,881) |
Pts (N=937) |
|||||
| Statement | Understand what it means (N=723) | Have heard of it (N=2,842) | Have never heard of it (N=7,316) | Understand what it means (N=123) | Have heard of it (N=292) | Have never heard of it (N=522) |
| Genome editing may be performed for diseases that shorten a baby's life | 32.9% | 25.9% | 20.3% | 30% | 20% | 20% |
| Genome editing may be performed for diseases that require long-term care | 56.1% | 61.0% | 51.7% | 52.6% | 44.0% | 36.6% |
| Not appropriate in any circumstances | 10% | 20% | 30% | 10% | 20% | 40% |
The survey looked at how much people know and accept human germline genome editing, and how worried they are about it. It found that people who understand what it means are more likely to accept it for serious diseases. Both patients (PTs) and the general public (GAs) have concerns about genome editing, especially about humans changing other humans' genes. In fact, PTs who understand genome editing are worried about it being used to change human genes, and GAs share similar concerns.

| Company Name | CRISPR Therapeutics |
| Headquarters | Zug, Switzerland |
| Pipeline |
The following list is of the genome editing pipeline:
|
| Company Name | Caribou Biosciences Inc. |
| Headquarters | California, U.S. |
| Pipeline | In April 2024, Caribou Biosciences, Inc. is a prominent clinical-stage CRISPR genome-editing biopharmaceutical company. The company announced that the FDA had approved its Investigational New Drug (IND) application for CB-010, an allogeneic anti-CD19 CAR-T cell therapy with a PD-1 knockout (KO), to treat extrarenal lupus (ERL) and lupus nephritis (LN). It is anticipated that by the end of 2024, a multicenter, open-label GALLOP clinical study including CB-010 in patients with LN and ERL would begin phase 1. |
By Application
By Technology
By Delivery Method
By End-use
By Region
December 2025
November 2025
November 2025
November 2025