November 2025

The global lentiviral vector market size is calculated at USD 360 million in 2024, grew to USD 426.82 million in 2025, and is projected to reach around USD 1943.24 million by 2034. The market is expanding at a CAGR of 18.56% between 2025 and 2034.

| Metric | Details |
| Market Size in 2025 | USD 426.82 Million |
| Projected Market Size in 2034 | USD 1943.24 Million |
| CAGR (2025 - 2034) | 18.56% |
| Leading Region | North America Share by 46% |
| Market Segmentation | By Product Type, By Indication, By End User, By Region |
| Top Key Players | Thermo Fisher Scientific Inc., Cobra Biologics Limited, Merck KGaA, Sirion-Biotech GmbH, OriGene Technologies Inc., FinVector Oy, Sino Biological Inc., Oxford Biomedica, Lonza, Cell Biolabs, GENEMEDI, Batavia Biosciences B.V., Cytiva, Takara Bio Inc., Sirion-Biotech GmbH (Revvity), Waisman Biomanufacturing, Vector Biolabs |
The research conducted on lentiviral vectors is increasing. At the same time, new gene therapies developed by using lentiviral vectors are moving towards clinical trials after successful preclinical trials. Furthermore, trials on beta thalassemia, AIDS, and Wiskott–Aldrich are also being conducted. Similarly, various animal models are also being treated with lentiviral vector-based gene therapy, suffering from cognate human diseases. The SIN lentiviral vectors also provided improved safety profiles over γ-RVs. Thus, with the help of lentiviral vectors, new, improved therapeutics are being used in the treatment of various incurable diseases.
AI plays an important role in the lentiviral vector market in the identification of genetic data, target sites, as well as it also enhances the safety and efficiency of the products developed using lentiviral vectors. The AI algorithm, as well as machine learning (ML), are used in detecting the gene sequence, or target sites, for enhancing the treatment. Moreover, it can also predict the risk involved with the treatment options, which in turn enhances the safety and efficiency of the products. Furthermore, it is also used in the production process, which reduces errors and increases the yield obtained.
Rising Awareness
Along with the increasing incidences of diseases, the awareness about early diagnosis as well as effective treatment approaches is increasing the demand for lentiviral vectors. At the same time, various awareness programs and campaigns are being conducted by governments and the private sector. This, in turn, increases the need for the use of personalized medications or gene therapies, which are developed with the help of lentiviral vectors. Furthermore, they are also being used in the production of various vaccines. Thus, this promotes the lentiviral vector market growth.
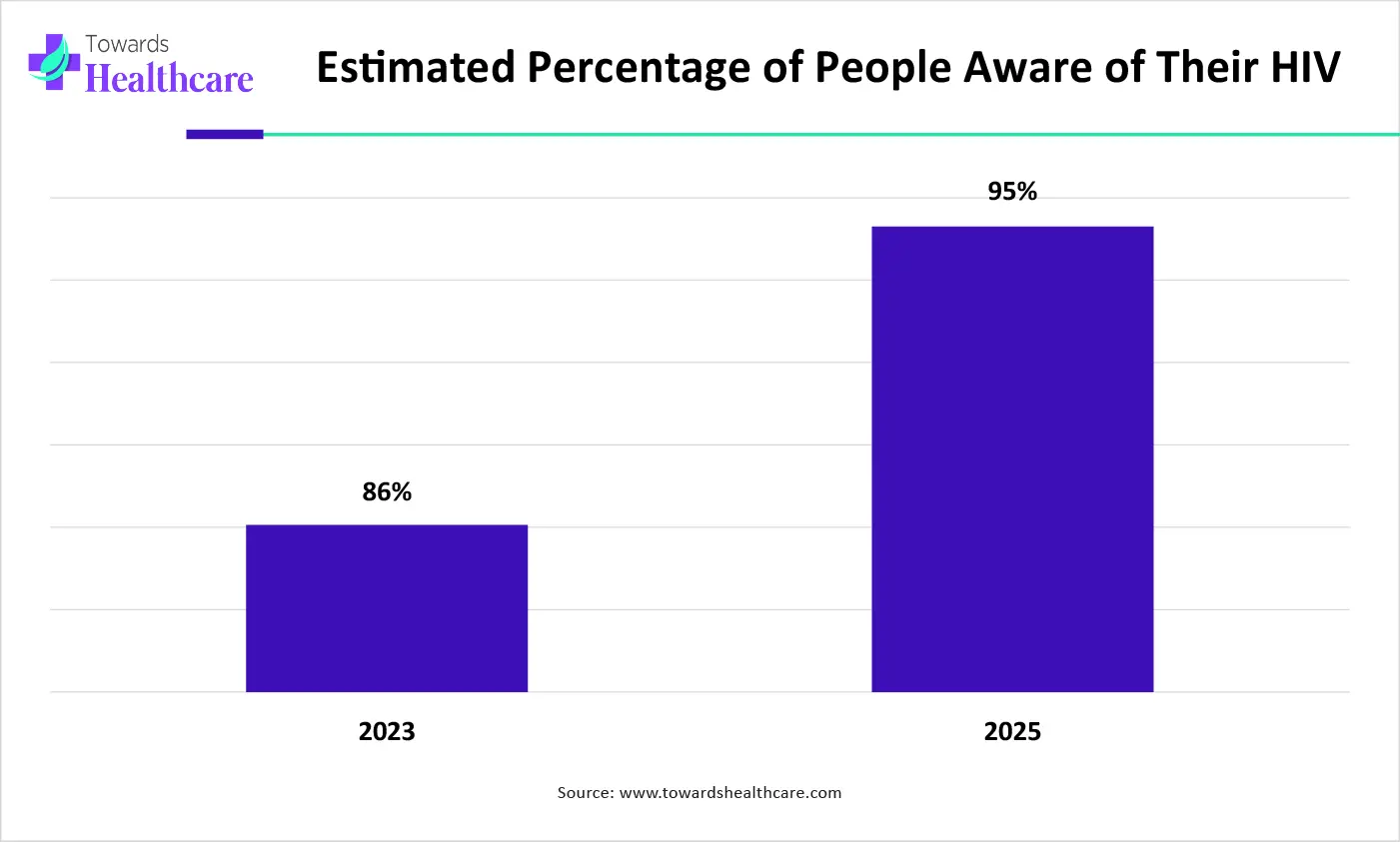
The graph represents a comparison between the estimated percentage of people aware of their HIV in year of 2023 and 2025. It indicates that there will be a rise in HIV awareness. Hence, it increases the demand for the use of lentiviral vector-based treatment options for the effective management of HIV. Thus, this in turn will ultimately promote the market growth.
High Cost
The lentiviral production process involves multiple steps as well as sophisticated equipment, which may be expensive. Moreover, the yield obtained is very low due to which makes it more costly. Hence, when these vectors are further used in the development of various therapies, the cost adds up. At the same time, the process involved in the clinical trials is also expensive. Thus, all these factors result in making the vectors expensive.
Increasing T-Cell Therapies
Due to the increasing genetic diseases, the demand for cell and gene therapies has increased. This, in turn, increased the use of lentiviral vectors for the development of these therapies. Thus, to enhance the targeted drug delivery by CAR-T cell therapies, lentiviral vectors are being used. Furthermore, it also increases the duration of action. Similarly, due to these advantages, it is also being used in the development of various other treatment approaches. Thus, all these factors increase the use of lentiviral vectors, which in turn also increase the research conducted. Hence, this promotes the lentiviral vector market growth.
For instance,
By product type, the 1st-generation segment dominated the market in 2024. The 1st-generation products were widely used in 2024, due to their enhanced stability, efficiency, as well as safety. This, in turn, contributed to the market growth.
By product type, the 3rd-generation segment is estimated to grow significantly at a notable CAGR during the forecast period. The 3rd-generation products are being used in the development of various cell and gene therapies due to their improved safety and stability.
By indication type, the HIV segment dominated the market in 2024. Due to increasing incidences of HIV, various research and development studies were conducted using lentiviral vectors. This enhanced the market growth.
By indication type, the Β-thalassemia segment is anticipated to grow significantly during the forecast period. Various new therapies are being developed with the help of lentiviral vectors for treating Β-thalassemia as they may target the defective gene.
By end user, the research institutes segment dominated the global lentiviral vector market in 2024. The research institutes were driven by the increased interest in the development of new lentiviral vector-based therapies. This promoted the market growth.
By end user, the hospital segment is predicted to be the fastest growing during the forecast period. The hospitals are providing lentiviral vector-based cell, gene therapies as well as personalized medications, accelerating the market growth.
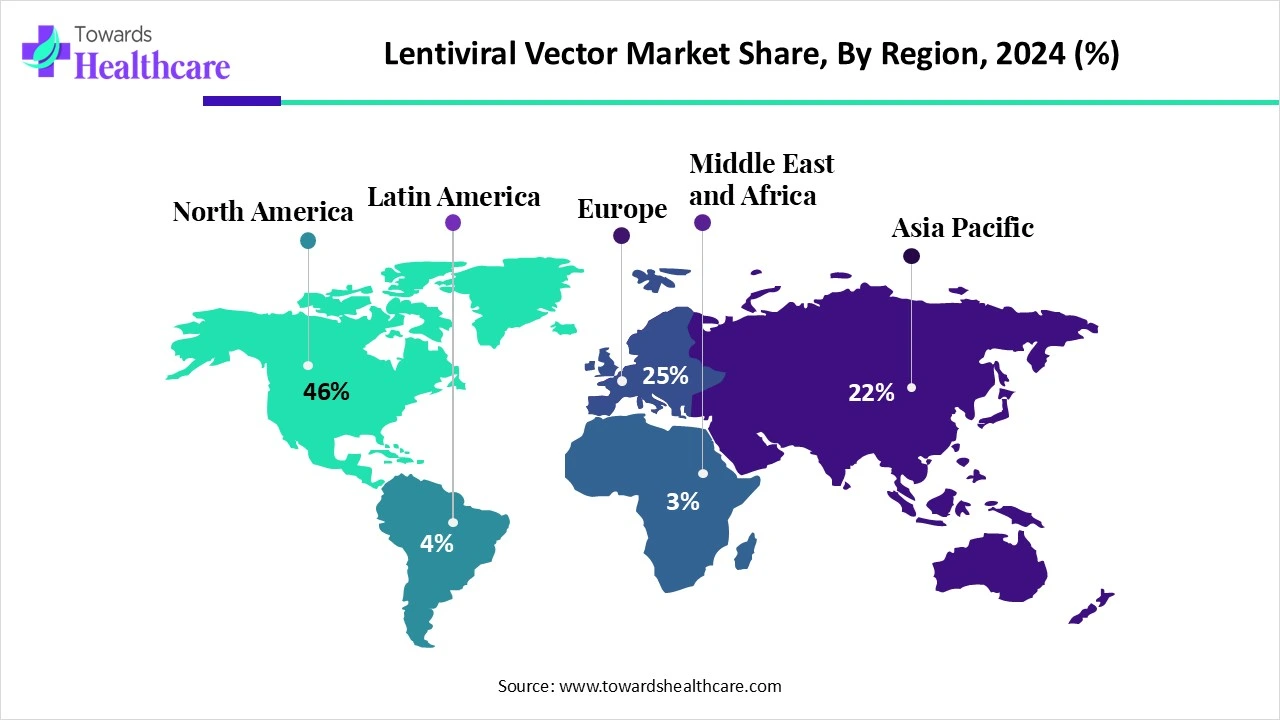
North America dominated the lentiviral vector market share by 46% in 2024. North America consisted of well-developed healthcare sectors with advanced technologies as well as skilled personnel. This, in turn, increased various treatment approaches by using lentiviral vectors. Thus, this contributed to the market growth.
The industries in the U.S are developing various new therapies with the help of lentiviral vectors for effective treatments of rising genetic diseases. At the same time, the government is also providing its support for making these treatment options easily accessible.
As the healthcare sector in Canada is advanced, it is using various technologies for conducting research for the development of new applications of lentiviral vectors. Furthermore, growing cancer cases are also increasing their demand, which leads to rising collaborations.
Asia Pacific is estimated to host the fastest-growing lentiviral vector market during the forecast period. Asia Pacific is experiencing a rise in disease occurrences in the population. Due to this, there is a rise in the demand for the use of personalized as well as other treatment options using lentiviral vectors. Thus, this is enhancing the market growth.
As China has a large population, the incidence of diseases also increases. Therefore, by using advanced technologies, the industries are developing new treatment approaches, as well as hospitals are also adopting various cell and gene therapies developed by lentiviral vectors.
The growing diseases in India are driving the demand for new personalized and gene or cell therapies. This increases the use of lentiviral vectors in their development. This is further supported by the government to make the treatment affordable.
Europe is expected to grow significantly in the lentiviral vector market during the forecast period. The industries as well as institutions in Europe are focusing on the development as well as detection of other applications of lentiviral vector use. Furthermore, the rule and regulations also enhance these workflows. Thus, this promotes the market growth.
For instance,
The industries in Germany are developing new therapies using advanced technologies, which is increasing the interest in institutes as well. At the same time, due to stringent regulations, the safety and efficiency of these therapies are also enhanced.
The institutes as well as the industries present in Germany are researching new applications of lentiviral vectors along with increased development of cell, gene therapies, and personalized medication. Moreover, these are supported by the government investments.

By Product Type
By Indication
By End User
By Region
November 2025
December 2025
October 2025
November 2025