
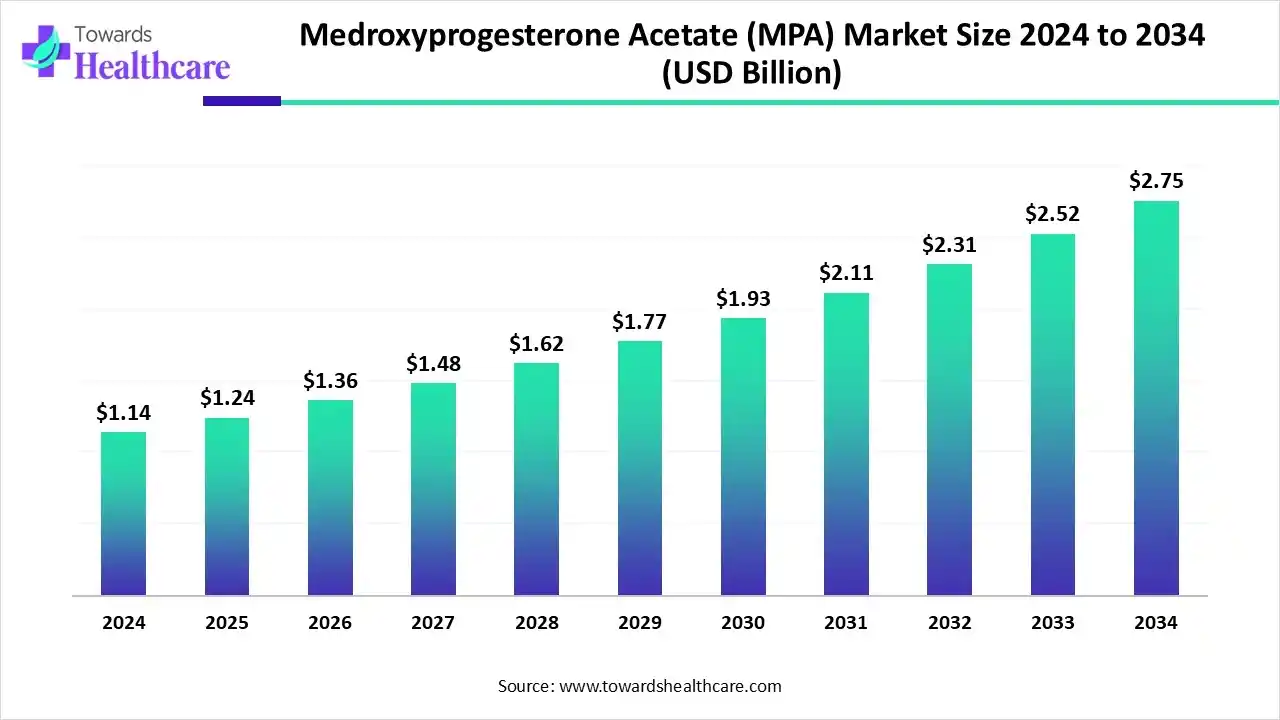
The medroxyprogesterone acetate market size was valued at US$ 1.14 billion in 2024 and is projected to grow to 1.24 billion in 2025. Forecasts suggest it will reach approximately US$ 2.75 billion by 2034, registering a CAGR of 9.13% during the period.
The medroxyprogesterone acetate market is witnessing steady growth, driven by rising cases of gynaecological disorders, increasing adoption of hormonal contraceptives, and expanding applications in hormone replacement therapy and cancer treatment. Advancements in long-acting injectables and improved drug delivery methods are further supporting market expansion. North America dominates the market due to its advanced healthcare infrastructure, high prevalence of reproductive health issues, strong pharmaceutical presence, and favourable regulatory environment. Growing awareness of women’s health and greater accessibility to treatments continue to strengthen regional demand.
The two major factors driving the growth of the medroxyprogesterone acetate (MPA) market are the rising prevalence of gynaecological disorders and the growing demand for effective contraceptive solutions. Medroxyprogesterone acetate is a synthetic progestin, a form of the female hormone progesterone, widely prescribed for several medical uses. It is commonly used in treating abnormal uterine bleeding, endometriosis, and menstrual irregularities. Additionally, MPA is a key component in long-acting injectable contraceptives and plays an important role in hormone replacement therapy for menopausal women. It is also prescribed in certain cancer treatments, including endometrial and renal cancers, expanding its therapeutic applications.
For instance,
The ultra-long-acting in-situ forming implant represents a significant advancement in the medroxyprogesterone acetate (MPA) market, combining contraceptive efficacy with improved patient convenience. Developed in 2024, this implant co-formulates MPA with antiretroviral drugs like dolutegravir or cabotegravir, providing dual protection against pregnancy and HIV. Its development aligns with global health priorities, especially in low-resource regions, offering a cost-effective, efficient, and discreet solution for reproductive health management while integrating modern biomedical technology for broader public health impact.
| Study ID | Study Title | Objective | Phase | Status | Region | Key Findings / Outcomes |
| NCT00064025 | Medroxyprogesterone in Advanced Cancer | Evaluate MPA's efficacy in advanced cancer | Completed | Completed | Global | Demonstrated potential in advanced cancer treatment |
| NCT06979596 | MK-5684 in Solid Tumors | Assess MK-5684's efficacy in solid tumors | Phase 2 | Not yet recruiting | Global | Aimed at evaluating efficacy in breast, ovarian, and endometrial cancers |
| ISRCTN62695528 | Contraceptive Efficacy Study | Evaluate the contraceptive efficacy and safety of DMPA | Phase 3 | Ongoing | Multi-center | Focus on depot medroxyprogesterone acetate (150 mg/ml) |
| TCTR20240628006 | Bone Marker Comparison Study | Compare the effects of DMPA and desogestrel on bone markers | Ongoing | Recruiting | Thailand | Investigating the bone health implications of contraceptive methods |
| JGOG2051/KGOG2031 | High-Dose MPA for Endometrial Cancer | Assess high-dose MPA therapy in recurrent endometrial cancer | Phase 2 | Ongoing | Japan | Evaluating efficacy and safety in early-stage endometrial cancer |
| NRG-GY011 | MPA and Entinostat in Endometrial Cancer | Investigate MPA plus entinostat in endometrial carcinoma | Pilot Study | Terminated | USA | Aimed at evaluating the treatment combination in endometrial cancer |
| NCT00064025 | Medroxyprogesterone in Advanced Cancer | Evaluate MPA's efficacy in advanced cancer | Completed | Completed | Global | Demonstrated potential in advanced cancer treatment |
The injectable segment dominates the market due to its convenience, long-acting effect, and high patient compliance. It provides reliable, sustained contraception, reduces dosing frequency, and ensures consistent therapeutic levels. Additionally, injectable formulations are widely used in hormone therapy and gynecological treatments, making them the preferred choice among healthcare providers and patients.
The combination injectable formulations segment is estimated to be the fastest-growing in the market due to rising demand for multi-purpose therapies that integrate contraceptives with other hormonal or antiretroviral agents. These formulations enhance convenience, improve patient compliance, and address multiple health needs simultaneously. Ongoing R&D and clinical trials are accelerating the adoption of these innovative combination therapies globally.
The contraception/family planning segment dominates the market due to widespread use of MPA in injectable contraceptives, high demand for long-acting reversible contraception, and growing awareness of family planning. Its effectiveness, safety, and ease of administration make it the preferred choice for women seeking reliable birth control solutions globally.
The endometriosis & polycystic ovary syndrome (PCOS) segment is anticipated to be the fastest-growing segment in the medroxyprogesterone acetate (MPA) market due to the increasing prevalence of these hormonal and reproductive disorders. Rising awareness among women and healthcare providers, coupled with MPA’s efficacy in regulating menstrual cycles, reducing pain, and managing hormonal imbalances, drives adoption. Ongoing research and development of targeted therapies further expand their clinical applications, accelerating market growth in this segment globally.
The intramuscular (IM) injection segment dominates the market due to its established efficacy, reliable absorption, and long-acting contraceptive effect. It ensures consistent therapeutic levels, reduces dosing frequency, and is widely preferred by healthcare providers for contraception and hormonal treatments, making it the most commonly used administration route globally.
The subcutaneous (SC) injection segment is estimated to be the fastest-growing segment in the medroxyprogesterone acetate (MPA) market due to its convenience, self-administration capability, and improved patient compliance. Unlike intramuscular injections, SC formulations use smaller needles, reduce pain, and allow at-home use, making them ideal for long-acting contraceptives and hormone therapies. Rising awareness, supportive clinical guidelines, and increased accessibility in developed and emerging markets have steadily driven adoption over the decade.
The women of reproductive age segment dominates the market due to high demand for effective contraceptives and hormone therapies in this group. MPA’s long-acting injectable formulations offer reliable birth control, menstrual regulation, and management of reproductive disorders. Increased awareness of family planning, accessibility to healthcare services, and government initiatives further reinforce its widespread adoption among women of reproductive age globally.
The post-menopausal women segment is estimated to be the fastest-growing segment in the market due to the rising need for hormone replacement therapy to alleviate menopausal symptoms such as hot flashes, mood swings, and bone density loss. MPA effectively regulates hormonal imbalances, reduces the risk of endometrial hyperplasia, and supports overall reproductive health. Increasing awareness, improved healthcare access, and supportive clinical guidelines are driving higher adoption, making this segment a key growth driver globally.
North America dominates the medroxyprogesterone acetate (MPA) market due to its advanced healthcare infrastructure, high awareness of reproductive health, and strong adoption of contraceptive and hormone therapies. The region benefits from well-established pharmaceutical companies, extensive distribution networks, and supportive regulatory frameworks that ensure accessibility of MPA products. Additionally, the prevalence of gynecological disorders, growing demand for long-acting contraceptives, and government initiatives promoting women’s health drive market growth. Continuous research, clinical trials, and innovations in formulations and delivery methods, such as long-acting injectables and combination therapies, further strengthen North America’s leadership and influence in the global market.
The U.S. leads the medroxyprogesterone acetate (MPA) market due to its advanced healthcare infrastructure, high awareness of reproductive health, and strong adoption of long-acting contraceptives and hormone therapies. Widespread availability of injectable formulations, supported by leading pharmaceutical companies, ensures accessibility across healthcare facilities. Rising prevalence of gynecological disorders, family planning initiatives, and government programs promoting women’s health further drive market growth.
Clinical trials and ongoing R&D for combination therapies and novel formulations strengthen innovation. Regulatory support, robust distribution networks, and patient education programs enhance uptake, making the U.S. the largest and most influential globally.
The Medroxyprogesterone Acetate (MPA) Market in Canada is expanding steadily, driven by increasing awareness of reproductive health and demand for effective contraceptive solutions. The country’s strong healthcare system, government-supported family planning programs, and insurance coverage ensure accessibility to injectable and long-acting MPA formulations.
Adoption is boosted by partnerships between pharmaceutical companies and public health initiatives, facilitating distribution and affordability. Clinical research and development of combination therapies and innovative drug delivery systems further broaden therapeutic options. Although smaller than the U.S., Canada shows consistent growth due to patient education, regulatory frameworks, and healthcare policies focused on improving women’s reproductive health outcomes nationwide.
The Asia-Pacific region is the fastest-growing in the medroxyprogesterone acetate (MPA) market due to increasing awareness of reproductive health, rising demand for long-acting contraceptives, and expanding healthcare infrastructure. Growing prevalence of gynecological disorders, government initiatives promoting family planning, and improved access to affordable MPA formulations drive adoption. Additionally, rapid urbanization, rising female workforce participation, and ongoing clinical research in countries like India, China, and Southeast Asia support the development and distribution of injectable and combination therapies, accelerating market growth across the region.
India’s medroxyprogesterone acetate (MPA) Market is expanding rapidly, driven by increasing demand for contraceptives and hormone therapies. Companies like Montage Laboratories are enhancing production capacities for MPA tablets, catering to both domestic and international markets. Government initiatives, improved healthcare access, and rising awareness of reproductive health further support market growth, making India a key contributor to the region’s MPA adoption.
China is investing heavily in hormone production, including MPA, to meet growing domestic and international demand. Recent funding rounds for multiple biopharmaceutical companies are enhancing production capabilities, enabling wider availability of MPA formulations. The focus on research, innovation, and efficient distribution networks supports rapid adoption of injectable and combination therapies across the country.
Japan emphasizes the development of hormone therapies, including MPA, through strategic investments in research and development. Regulatory frameworks, such as requirements for Marketing Authorization Holder and Accreditation of Foreign Manufacturer status, ensure high-quality and compliant MPA products. Ongoing innovation, clinical research, and increasing adoption of long-acting injectables and combination therapies strengthen Japan’s position in the regional market.
Key Organizations:
Key Organizations:
Pfizer
Teva Pharmaceuticals
Mylan Pharmaceuticals (now part of Viatris)
Montage Laboratories Pvt. Ltd.
Remedial Healthcare
Amgen
Eugia Pharma
By Dosage Form
By Application/Indication
By Route of Administration
By End-User/Patient Type
By Region
