December 2025

The nanomedicine market size is anticipated to grow from USD 265.88 billion in 2025 to USD 632.09 billion by 2034, with a compound annual growth rate (CAGR) of 10.1% during the forecast period from 2025 to 2034. The growing research and development activities and advancements in technology boost the market.
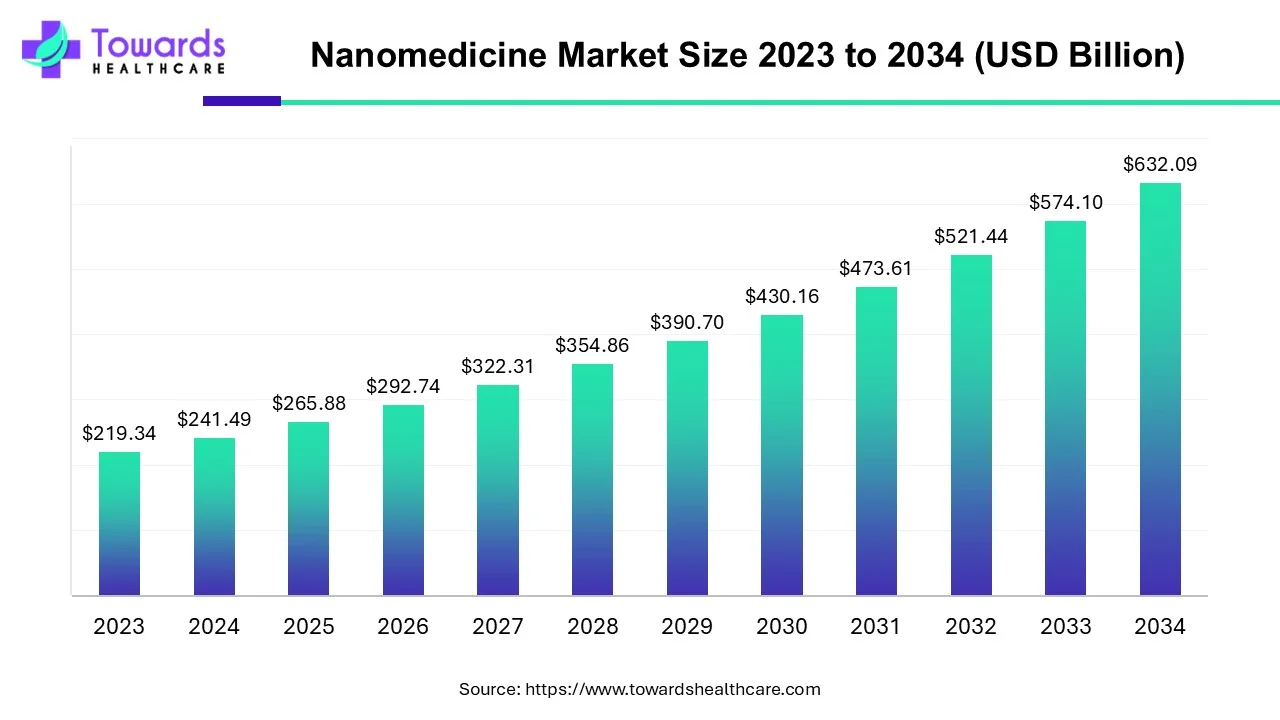
According to recent data, around 100+ nanomedicines have been commercially marketed, with almost 550+ nanomedicines under clinical trials.
Nanomedicine involves creating and utilizing tiny structures or devices, typically between 1 and 100 nanometers in size, for medical purposes such as diagnosis and treatment. These nanoscale objects or tools, including nano-robots, skin patches, or other nanostructured materials, leverage their unique properties to achieve specific medical effects.
In medicine, nanomedicine holds promise for better drug delivery systems, more accurate imaging techniques, and even tiny devices that can target and treat diseases directly at the cellular level. Electronics enables the development of smaller and faster devices, leading to better computers, smartphones, and other gadgets.
Nanomedicine is increasingly used to treat a wide range of diseases due to its ability to target specific cells or tissues precisely. In cancer treatment, nanomedicine delivers chemotherapy drugs directly to cancer cells, reducing side effects and improving effectiveness. It's also used in imaging techniques like MRI and CT scans to detect tumors early.
Additionally, nanomedicine is being explored for treating neurological disorders such as Alzheimer's and Parkinson's by delivering drugs across the blood-brain barrier and targeting diseased cells. In infectious diseases like HIV/AIDS and tuberculosis, nanomedicine can enhance drug delivery and improve the efficacy of antiviral or antibiotic medications. Furthermore, nanomedicine is being investigated for treating cardiovascular diseases, diabetes, and autoimmune disorders by developing targeted drug delivery systems and implants that regulate blood sugar levels or modulate immune responses.
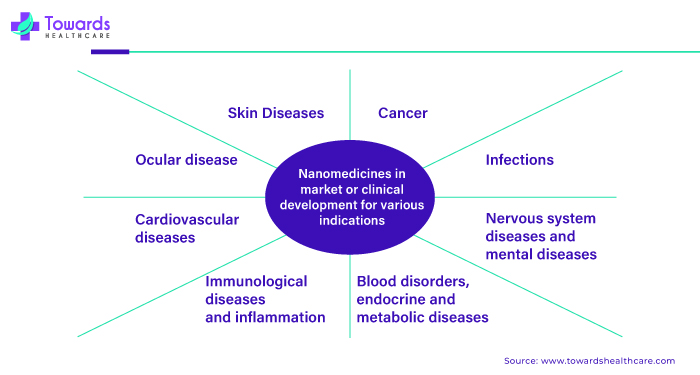
Nanomedicine holds great promise in revolutionizing the treatment of various diseases by providing more effective, targeted, and less invasive therapies, potentially improving patient outcomes and quality of life.
Artificial intelligence (AI) can play a vital role in developing novel nanomedicines with desired therapeutic effects. AI can also suggest the type of nanomedicines to be used for specific drug delivery systems with desired properties. AI and machine learning (ML) algorithms can predict the physicochemical, pharmacokinetic, and pharmacodynamic properties of nanomedicines. This helps researchers to develop drugs with enhanced efficiency and reduced side effects. Technological advancements, such as AI-based sensors, enable healthcare professionals to control and adjust doses via a mobile application. This leads to targeted and modified delivery of nanomedicines within the body. Moreover, AI can predict the treatment outcomes of nanomedicines in real time, allowing for more precise adjustments to treatments.
Because cancer is becoming more common, we need new and better treatments. Nanomedicine is one promising way to fight cancer. It works by delivering treatments directly to cancer cells, which can make them more effective and cause fewer side effects. As more people get cancer around the world, scientists are seeing how nanomedicine could change the way we treat it. By using minimal materials and methods, researchers hope to improve cancer treatments and ease the disease's burden on society.
The rising number of individuals with cancer has led to significant growth in the nanomedicine market. This is because nanomedicine has some cool ways to help treat cancer. It helps deliver cancer drugs to the cancer cells so they work better and have fewer side effects. It improves how we see cancer using fancy imaging techniques like MRI and CT scans. This helps doctors find cancer early and treat it sooner.
Biomarker detection using nanomedicine is a fancy way of saying that tiny devices and sensors can find signs of cancer in our body fluids, like blood or urine. These signs, called biomarkers indicate whether cancer is present. These nanomedicine-based devices are super sensitive and accurate to spot even tiny amounts of these biomarkers. This helps doctors find cancer early when it's easier to treat and has a better chance of being cured.
Not only can these nanodevices detect cancer, but they can also keep an eye on how the disease is changing over time. This helps doctors track treatment progress and see if it's working well. Plus, by predicting how cancer might respond to different treatments, these nanomedicine-based tools can help personalize treatment plans for each patient. Biomarker detection using nanomedicine is a powerful tool for early cancer detection, monitoring disease progression, and guiding treatment decisions, all of which can improve outcomes for cancer patients.
Nanomedicine lets us make personalized treatments for each person's unique cancer. That means better results and fewer problems from treatment. It helps develop new cancer therapies like heat and light therapy and boosts the body's immune system to fight cancer. Nanomedicine can spot tiny signs of cancer in our body fluids, which helps catch it early and track how it's doing during treatment. So, because more people have cancer, there's a more significant need for these intelligent nanomedicine solutions to help diagnose, treat, and keep an eye on cancer, which is making the nanomedicine market grow.
Alzheimer's disease ranked as the sixth most common cause of death in the United States, and it dropped to seventh place in both 2020 and 2021. Neurological disorders like Alzheimer's disease are becoming more common, and nanomedicine offers promising ways to manage them. In Alzheimer's, for example, the brain gets clogged with clumps of proteins, leading to memory loss and cognitive decline. Nanomedicine can help by delivering drugs directly to these protein clumps, breaking them apart and slowing down the progression of the disease.
Additionally, nanoparticles can carry drugs across the blood-brain barrier, a protective layer around the brain that usually prevents medications from getting in. This allows for more effective treatment of neurological disorders.
Nanomedicine also enables the development of advanced imaging techniques to detect brain changes associated with neurological diseases early, allowing for timely intervention and treatment. Nanomaterials can create implants or devices that stimulate or regulate brain activity, relieving symptoms and improving the quality of life for patients with neurological disorders.
Furthermore, researchers are exploring using nanotechnology in regenerative medicine to repair damaged nerve cells and promote brain tissue regeneration. By harnessing the unique properties of nanomaterials, scientists are optimistic about the potential of nanotechnology to revolutionize the management of neurological disorders like Alzheimer's, offering hope for improved outcomes and quality of life for patients and their families.
Continuous improvements in making tiny things, studying them, and simulating their behavior are pushing nanotechnology forward and making the market grow. Nanofabrication techniques are methods for building small structures, like using special machines to create tiny devices or materials. Characterization tools help us understand what these little things are made of and how they work by analyzing their properties. Simulation software allows scientists to predict how nanomaterials will behave under different conditions without physically testing them.
All these advancements are opening up new possibilities for nanomedicine, making creating innovative products and solutions easier. For example, better nanofabrication techniques allow us to make smaller, more precise devices, while improved characterization tools help us understand how nanomaterials interact with living cells or the environment. This helps researchers develop new nanotechnology-based products for various industries, from electronics and healthcare to energy and environmental sustainability. As a result, the nanotechnology market is growing as more companies invest in developing and commercializing these advanced technologies.
Getting approval for nanomedicine products can be tricky because nanoparticles behave differently than regular medicines. This makes it hard for regulators to determine their safety and effectiveness. To make things easier, we need to set up standard ways to test nanomedicine and transparent rules for how they should be regulated. This would help speed up the approval process and make it easier for these products to enter the market. Without these guidelines, it's tough for companies to get their nanomedicine products approved, which slows progress and makes it harder for patients to access these innovative treatments. Setting up clear rules and testing methods for nanomedicine is essential for getting these products out to those needing them.
| Nanosystem Type | Product Name | Active Ingredient(s) | Company | Indication(s) |
| Liposome | Myocet | Doxorubicin hydrochloride and an anthracycline cytotoxic agent | Teva Pharmaceutical Industries Ltd. | Metastatic breast cancer |
| Liposome | Onivyde | Irinotecan | Merrimack Pharmaceuticals | Metastatic pancreatic cancer |
| Nanoemulsion | Restasis | Cyclosporine | Allergan | Chronic dry eye |
| Micelle | Apealea | Paclitaxel | Oasmia Pharmaceutical AB | Ovarian cancer, peritoneal cancer, fallopian tube cancer |
| Hafnium oxide nanoparticles | Hensify | Hafnium oxide | Nanobiotix | Locally advanced squamous cell carcinoma |
By application, the drug delivery segment held a dominant presence in the market in 2024. The rising prevalence of chronic disorders and their increasing complexity necessitate researchers to develop novel and more advanced drug delivery systems. Nanomedicine is an emerging field for targeted delivery using drug delivery systems. It acts as a drug carrier that encapsulates drugs and delivers them to specific locations in the body. It can increase the stability and solubility of biological molecules and modify their surfaces. Leveraging nanomedicines in drug delivery systems also improves drug bioavailability and absorption time.
By application, the therapeutics segment is expected to grow at the fastest rate in the market during the forecast period. Nanomedicines are widely used as therapeutics for various purposes, such as vaccines, immunotherapy, gene delivery, and tissue engineering. The growing demand for personalized therapy augments the segment’s growth. Nanomedicines have the potential to enhance their effectiveness and minimize systemic exposure and side effects. They have been widely studied for the treatment of cancer and other related disorders.
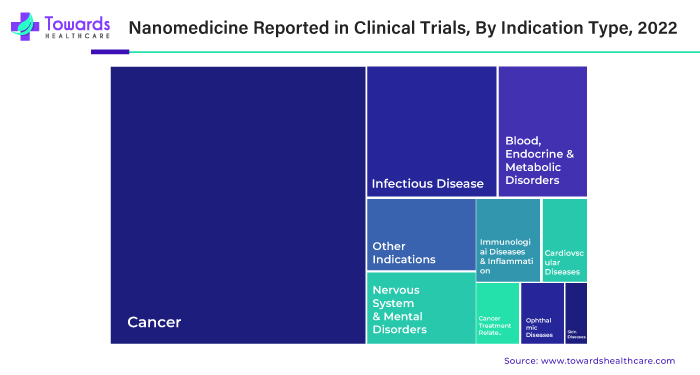
By indication, the clinical oncology segment led the global market in 2024. The rising prevalence of different types of cancer fuels the segment’s growth. The American Cancer Society estimates that there will be more than 2.04 million new cancer cases and 0.6 million cancer-related deaths in the U.S. in 2025. Nanomedicines are studied for their role in cancer diagnosis and treatment. Some researchers have found that they can modulate the biodistribution and target site accumulation of chemotherapeutic drugs. The U.S. FDA has approved at least 15 cancer nanomedicines, and around 80 are under 200 clinical trials.
By indication, the infectious disease segment is anticipated to grow with the highest CAGR in the market during the studied years. The increasing incidences of infectious diseases contribute to the segment’s growth. The demand for nanomedicines for treating infectious diseases is increasing due to the rise of drug resistance and the need for targeted therapeutics. Nanomedicines act as adjuvants and vaccine delivery vectors to enhance vaccine-induced specific immune responses.
By molecule type, the nanoparticles segment held the largest share of the market in 2024. Nanoparticles are nanomaterials 1 to 100 nm in diameter. They can be used for testing biomolecules as biomarkers and tumor labels. They are also widely used in smart pills that control a medication dose release depending on data collected throughout the body. They can also enhance the diagnostic accuracy of imaging techniques, such as PET or ultrasound, by enhancing fluorescent imaging. They are studied for the designing and manufacturing of novel scaffold structures for tissue and bone repair.
By molecule type, the nanotubes segment is predicted to witness significant growth in the market over the forecast period. A nanotube is a cylindrical structure with a hollow core, typically composed of carbon atoms. Nanotubes have been proven to be excellent vehicles for drug delivery. The growing demand for regenerative medicines propels the segment’s growth. Nanotubes are also used for biosensor diagnosis, enantiomer separation of chiral drugs, and extraction and analysis of drugs and pollutants.
North America, including the United States, Canada, and Mexico, plays a significant role in nanomedicine. In the United States, many research institutions and companies work on nanomedicine. Major pharmaceutical companies invest much in nanotechnology for medicines, diagnostics, and treatments. The FDA makes sure nanomedicine products are safe and work well. Canada also does a lot of nanomedicine research. Universities, government, and companies work together on new ideas. Canadian companies focus on using nanotechnology to deliver drugs and make better medical images. Rules in Canada make sure nanomedicine is developed and used responsibly. The North American nanomedicine market is growing because of solid funding, rules, and healthcare. More people have long-term illnesses, so there's a more significant need for new treatments. Better technology, like new ways to deliver drugs and diagnose diseases, is also helping. When academics, businesses, and government work together, it improves nanomedicine and helps it grow in North America.
The U.S. government manages work on nanotechnology by 19 government agencies through the National Nanotechnology Initiative (NNI). Encourage basic research to achieve fundamental understanding and knowledge of nanoscale processes and phenomena. It offers mechanisms to enable the transfer of technology in commercial applications and to support basic and applied research, which drives the growth of the market.
Countries like China, Japan, India, and South Korea are big players in nanomedicine in the Asia-Pacific region. This conference aimed to share scientific advancements, industry developments, technical progress, and emerging challenges within the field of nanotechnology. They have many research centers and companies developing new nanomedicine products. Governments support these efforts with funding and initiatives. With growing healthcare needs and investments, the region is seeing rapid advancements in nanotechnology for medicine. Collaboration between countries is also helping to drive innovation and improve healthcare options.
China potentially invested in nanoscience, establishing industry hubs, research centers, and global conferences to drive commercialization and innovation. Strong Presence of healthcare initiatives and organizations shaping China's nanotechnology sector. This sector majorly focuses on the medium and long-term nanotechnology programmes, which drive the growth of the market.
Jeremie Trochu, CEO of Ardena, commented that achieving full GMP approval underscores the company’s commitment to equipping clients with world-class capabilities for the development and manufacturing of complex nanomedicine formulations. He also said that he is proud of his team’s dedication to maintaining the highest quality and regulatory standards.

By Application
By Indication
By Molecule Type
By Geography
December 2025
November 2025
November 2025
February 2026