Explore Biopharma Companies with Trends Analysis and Market Growth

- Abbott Laboratories
- Amgen, Inc.
- Biogen, Inc.
- Eli Lilly and Company
- F. Hoffmann-La Roche, Ltd.
- Johnson & Johnson
- Merck & Co., Inc.
- Novo Nordisk A/S
- Pfizer, Inc.
- Sanofi
Biopharmaceuticals Market Overview
A biopharmaceutical, also known as a "biologic," is a medical therapeutic (or drug) derived from a biological source, such as a cell. Biotechnology is used to directly extract proteins and nucleic acids from biological sources to produce biopharmaceuticals, which are drugs used in medicine. Transgenic organisms are genetically modified plants and animals that are used in the production of biopharmaceuticals, which are pharmaceuticals made primarily from living organisms. This controversial technology is still in the trial stage.
Market Growth
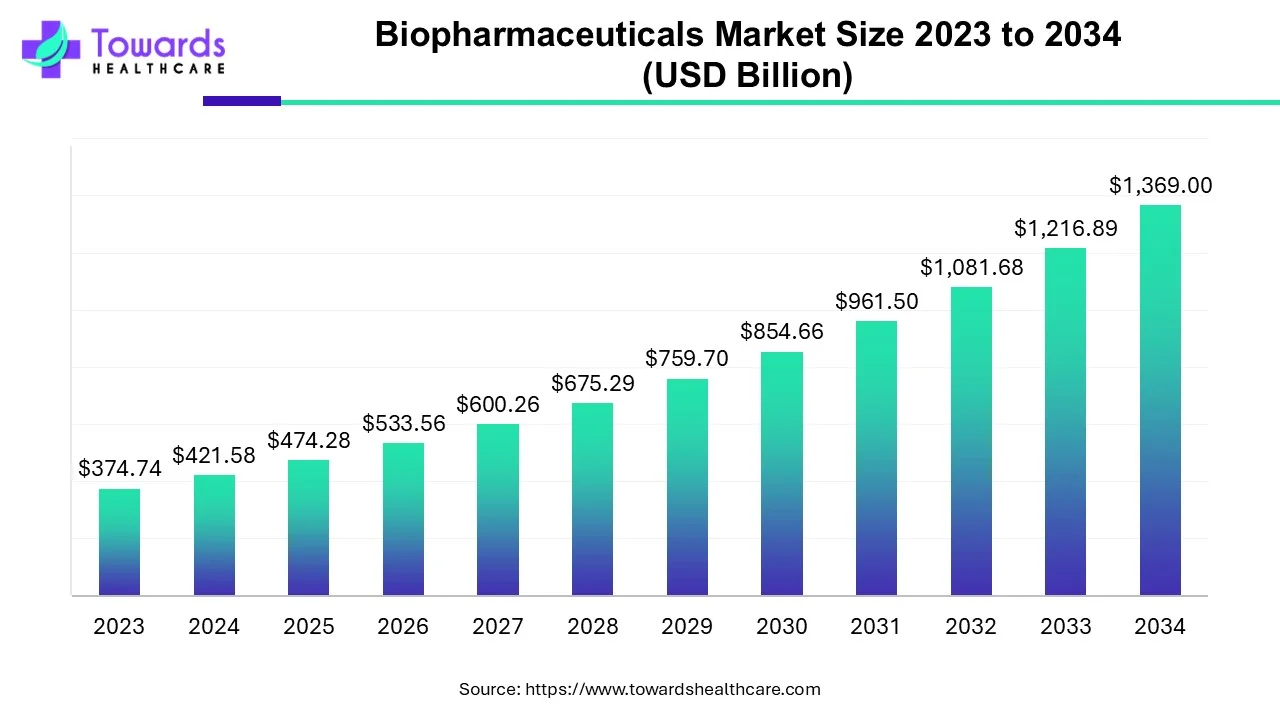
The global biopharmaceuticals market size was calculated at USD 421.58 billion in 2024, to reach USD 474.28 billion in 2025 is expected to be worth USD 1369 billion by 2034, expanding at a CAGR of 12.5% from 2024 to 2034.
Biopharmaceuticals Market Trends
- In January 2025, Takara Bio announced an acquisition of ViSpot to strengthen its biopharma services. The acquisition will enhance Takara Bio’s quality testing capabilities and expand its CDMO services.
- In December 2024, Ikena Oncology, Inc. announced a merger agreement with Inmagene Biopharmaceuticals for a $75 million private placement. The collaboration was made to develop IMG-007, a monoclonal antibody targeting OX40 for the treatment of atopic dermatitis.
- In October 2024, Emcure Pharmaceuticals announced the launch of Emcutix Biopharmaceuticals Limited focused on innovative dermatology solutions. The facility was launched to provide a differentiated portfolio to the unmet needs of the Indian dermatology market.
Expanding Biopharma Innovation
The biopharma industry is experiencing a golden era of innovation, marked by its largest and most diverse clinical pipeline in history, a culmination of decades of groundbreaking research. The biopharma research and innovation is driven by increasing funding from government and private institutions. This leads to the development of an expansive pipeline of drugs by various pharmaceutical and biotechnology companies.
Government funding includes an estimated $18 billion for Operation Warp Speed and $48 billion annually from the National Institutes of Health. Additionally, the private sector has contributed significantly, with an estimated $146 billion in life sciences venture capital funding over the past three years.
Biopharmaceuticals Market Competitive Landscape
One of the most important goals of biopharmaceutical innovation is to contribute to lower mortality and premature death rates. The Manhattan Institute recently published a research study on the reasons why the average life expectancy and longevity vary from country to country. The effective contribution of new bio drugs demonstrates that the more we use new bio drugs, the more we gain longevity and provide welfare to the population. Furthermore, tailoring drug formulations to specific patients and cases leads to improved cures and reduce premature mortality, both of which are important public health goals. Since the biopharmaceutical field was further revolutionized and initiated, to develop and discover new bio drugs, we have observed that this contributes to an increase in average life expectancy6, indicating a significant impact.
Bio-pharma firms can create drugs from biological components of substances known as biologics, which include sugars, proteins, nucleic acids such as DNA and RNA, and complex combinations of components. Biologics, or their precursors or components, are isolated from living sources such as humans, animals, plants, fungi, or microbes. Because they are structurally identical, these drugs are frequently more effective and have fewer side effects.
The majority of biopharmaceuticals are derived from naturally occurring proteins. Biopharmaceuticals are also derived from recombinant proteins, which are special proteins that contain genes that scientists have manipulated. The changes cause the proteins to behave in a specific manner; because the changes are encoded in the genes, each subsequent generation of proteins carrying the modified genes will behave in the same manner.
Latest Announcement by Industry Leaders
Ray Mercier, President of Thermo Fisher Scientific’s single-use technologies business, commented on the launch of bio-based films that biopharma manufacturers are focused on maximizing productivity, accelerating time-to-market, and generating efficiencies along with improving the overall sustainability of their operations. The bio-based films developed by the company allow customers to reduce their environmental impact.
Recent Developments
- In September 2024, Axio BioPharma unveiled its protein manufacturing services to meet the growing demand of the biopharmaceutical industry. The facility is accepting recombinant protein manufacturing projects in Madison, Wisconsin.
- In March 2024, Gennova Biopharmaceuticals announced the launch of a pediatric pack of pegaspargase, Hamsyl-Junior, for the treatment of rare blood cancer. The pack will be available in a 1500 IU pack, priced at Rs. 20,970.
Collaborate with our experts to explore the Biopharmaceuticals Market at sales@towardshealthcare.com
Keypoints
- Company Overview
- Locations Subsidiaries/Geographic reach
- Key Executives
- Company Financials
- Patents registered
- SWOT Analysis
- Applications Catered
- Strategic collaborations
- Recent Developments
- Competitive Benchmarking
