Learn About the Top U.S. Cell Therapy Market Companies
- Aurion Biotech
- Vertex Pharmaceuticals Incorporated
- Cellular Biomedicine Group, Inc
- Nkarta, Inc
- Atara Biotherapeutics, Inc
- Johnson & Johnson
- CARGO Therapeutics, Inc.
- Bristol-Myers Squibb Company
- Selecta Bioscience
- Gilead Sciences, Inc.
Industry at a Glance
The U.S. cell therapy market encompasses research & development, therapeutics, medication, education, equipment, software, and different resources associated with cell therapy. Cell therapy is a type of treatment where a patient receives an injection, graft, or implant of live cells in order to have a therapeutic effect. It is also known as cellular therapy, cell transplantation, or cytotherapy. The most widely used and proven cell transplantation therapy is bone marrow transplants. Treatments for cancer, autoimmune illnesses, infections, urinary issues, spinal cord injuries, rebuilding damaged joint cartilage, strengthening immunocompromised people, and neurological disorders are among the potential uses of cell treatments. Hospitals, clinics, biotechnology, research & development, pharmaceutical, and educational sectors are among the main industries contributing to the growth of the U.S. cell therapy market. The future growth of the market is being aided by research and development, treatment customization, and technological improvements.
Market Growth
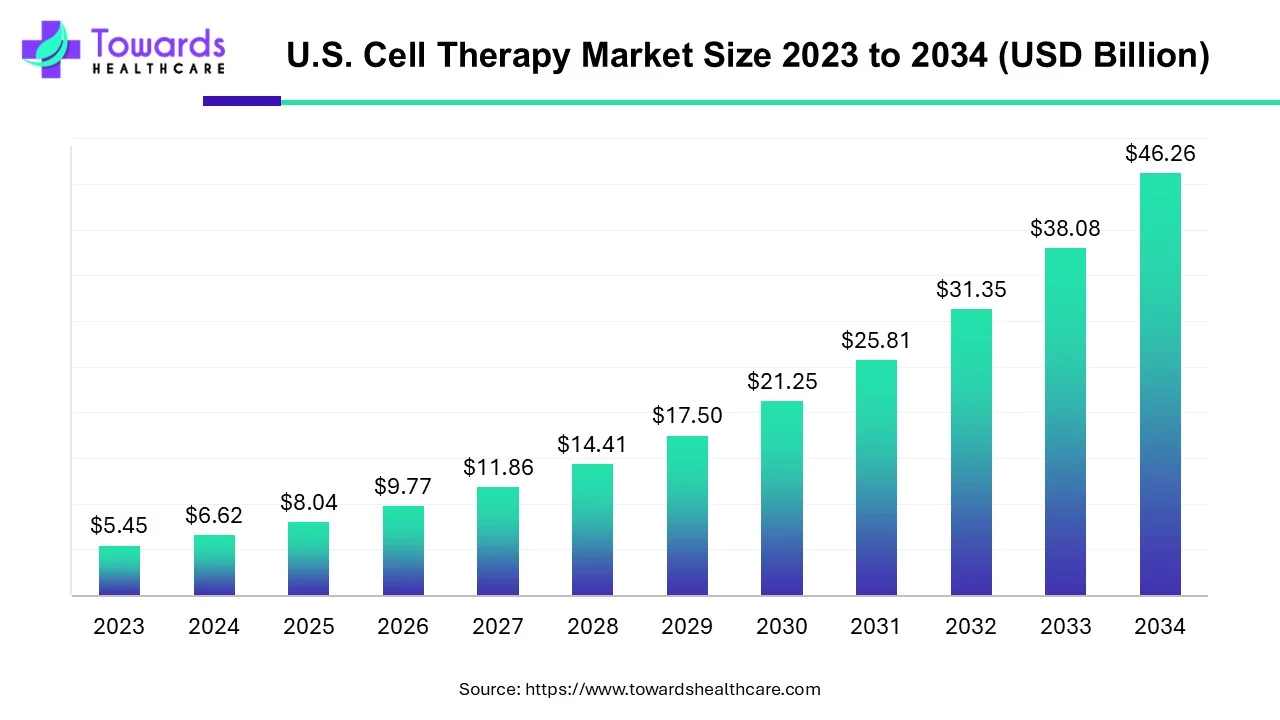
The U.S. cell therapy market size is forecast to grow at a CAGR of 21.46%, from USD 8.04 billion in 2025 to USD 46.26 billion by 2034, over the forecast period from 2025 to 2034.
Market Trends
- In April 2025, as per the 2 different clinical trials, stem cell therapies are considered a safe and effective way for the treatment of Parkinson's disease. The study used cells derived from human embryonic stem cells and human induced pluripotent stem cells, which were mentioned in the papers published in the Nature journal.
- In April 2025, lab-grown immune cells were developed by the researchers of the University of California, Irvine. It can track and clear the toxic brain buildup, which helps in restoring memory as well as brain function in mice. This was achieved by developing brain immune cells called microglia using stem cells (that developed into any type of body cell).
- In April 2025, as per the researcher from The University of Texas MD Anderson Cancer Center, significant results were obtained along with an acceptable safety profile in patients suffering from two types of advanced thyroid cancer using AIC100, which is a new chimeric antigen receptor (CAR) T cell therapy.
- In April 2025, a new CAR-T cell therapy targeting the CD30 protein (HSP-CAR30) was developed by the collaboration between the researchers from Sant Pau Research Institute (IR Sant Pau), Sant Pau Hospital, and the Josep Carreras Leukaemia Research Institute, which showed high efficacy in patients with refractory CD30+ lymphoma. According to the results of the Phase I clinical trial published in the prestigious journal Blood, stated that long-lasting responses along with enhanced clinical outcomes in treated patients are promoting the expansion of memory T cells by this new CAR-T30 therapy.
- In April 2025, the EchoBack CAR T-cell, which is a newly developed cancer-fighting immune cell, was formulated by the USC researchers. It utilizes focused ultrasound that helps to activate and sustain a powerful attack targeted at the tumors. These cells can remain effective for various days without tiring, and they only activate around the cancer cell, which in turn minimizes the damage to the healthy tissue.
Companies Insights
- In June 2024, in order to enable pharmaceutical companies and bigger healthcare providers to produce chimeric antigen receptor T (CAR T) cell therapies and other cell-based treatments more quickly and affordably, Cytiva introduced its Sefia next-generation manufacturing platform. In order to create Sefia, Kite, a Gilead firm, collaborated with Cytiva utilizing both companies' R&D and manufacturing capabilities as well as Kite's experience developing two popular CAR T treatments, Tecartus® (brexucabtagene autoleucel) and Yescarta® (axicabtagene ciloleucel).
- In March 2024, the Blood and Marrow Transplantation (BMT) and Cell Therapy Program at City of Hope Cancer Center Atlanta was launched, according to a statement from City of Hope®, one of the biggest cancer research and treatment organizations in the U.S.. One of the biggest and most effective transplant programs in the U.S., the City of Hope was a pioneer in BMT and has performed close to 19,000 successful transplants since the program's founding in 1976. This spring, the City of Hope Atlanta will perform its first autologous stem cell transplant surgery and offer BiTE treatment.
- In April 2024, in the U.S., VetStem, Inc. introduced the first allogeneic off-the-shelf cell medication product to be evaluated by the FDA. This leucoreduced, allogeneic, pooled, freeze-dried PRP is meant to offer a concentrated platelet in plasma source unique to canines for intra-articular injection. The horse version is expected to pass FDA scrutiny early in May and go on sale the same month.
Company Revenue
- In July 2025, Johnson & Johnson released their revenue reports, which showed adjusted earnings of $2.77 per share for the second quarter of 2025, with 1.8% year-over-year growth. Furthermore, a growth in sales was observed with $23.74 billion compared to the consensus of $22.85 billion, up to 5.8% year over year. Additionally, the operational growth and adjusted operational growth were noted to be 4.6% and 3%, respectively. Moreover, a rise of 4.9% or 3.8% operationally to $15.20 billion was reported by the sales of innovative medicines.
- In March 2025, the fourth quarter financial results and year ended for December 31, 2024, were released by Nkarta, Inc., which is a clinical-stage biopharmaceutical company developing engineered natural killer (NK) cell therapies. The marketable securities as of 31st December 2024, including investments, cash, cash equivalents, and restricted cash, were noted to be $380.5 million.
- $23.1 million and $96.7 million were the R&D expenses reported for the fourth quarter of 2024 and for the full year 2024, respectively. Similarly, the general and administrative (G&A) expenses were noted to be $7.8 million and $31.5 million for the fourth quarter of 2024 and the full year 2024, respectively.
- In February 2025, the fourth quarter financial results and year ended for December 31, 2024, were released by Vertex, Inc. The total revenues showed a rise of 15.2% year-over-year increase with $178.5 million for Q4 and a 16.5% increase to $666.8 million for the full year.
- Strong operational cash flow was achieved with an adjusted EBITDA of $38.1 million and non-GAAP net income of $25.5 million. While 16.8% rise for Q4 and 27.0% for the full year were reported, highlighting the significant growth in software subscriptions and cloud revenues. Moreover, revenue growth between $760 million and $768 million will be the goal of the company for 2025.
Latest Announcements by Industry Leaders
In August 2025, after announcing a $100,000 G-Rex® Grant, the Research Investigator at Sidra Medicine, M.D., Ph.D. Sara Deola commented that innovations in precision medicine are the main focus of Sidra Medicine, which is a pioneering healthcare institution. For pediatric diseases like cancers, rare and genetic diseases, this GMP facility provides better treatment protocols. Thus, with this grant, the protocols will be accelerated, specifically accelerating the pediatric oncology program of Sidra Medicine. Moreover, the G-Rex® method is considered to be outstanding, as it will provide a cell manufacturing platform suitable for T cell phenotype, promoting pediatric patient care and cure.
Recent Developments
- In April 2024, As the firm increases its core pharmacy business, Walgreens is investing in its capabilities and extending its services for specialty pharmacies in an effort to enhance patient outcomes and give payers and partners more value. Today, the firm unveiled Walgreens Specialty Pharmacy, a comprehensive service that increases patients' access to care for chronic, complicated illnesses and facilitates collaborations that boost the profitability of Walgreens' pharmacy division. Additionally, the business is investing in areas that will change the services it offers as a specialist pharmacy, such as gene and cell therapy.
- In March 2024, for children under the age of eighteen who have the most prevalent kind of lupus, Seattle Children's has been given permission by the FDA to begin the nation's first clinical trial with chimeric antigen receptor (CAR) T cells. Launching this first-in-country experiment might provide patients with treatment that could change their lives, which is why Seattle Children's is delighted.
- In November 2022, the UC San Diego Alpha Stem Cell Clinic received $8 million from the California Institute for Regenerative Medicine (CIRM) as part of a state-wide initiative to improve stem cell therapy. The money will help the clinic fulfill its goal of providing patients with illnesses that are challenging to cure with novel stem cell-based treatments.
Partner with our experts to explore the U.S. Cell Therapy Market at sales@towardshealthcare.com
Keypoints
- Company Overview
- Locations Subsidiaries/Geographic reach
- Key Executives
- Company Financials
- Patents registered
- SWOT Analysis
- Applications Catered
- Strategic collaborations
- Recent Developments
- Competitive Benchmarking
