U.S. Cell Therapy Market Size, Trends and Key Players
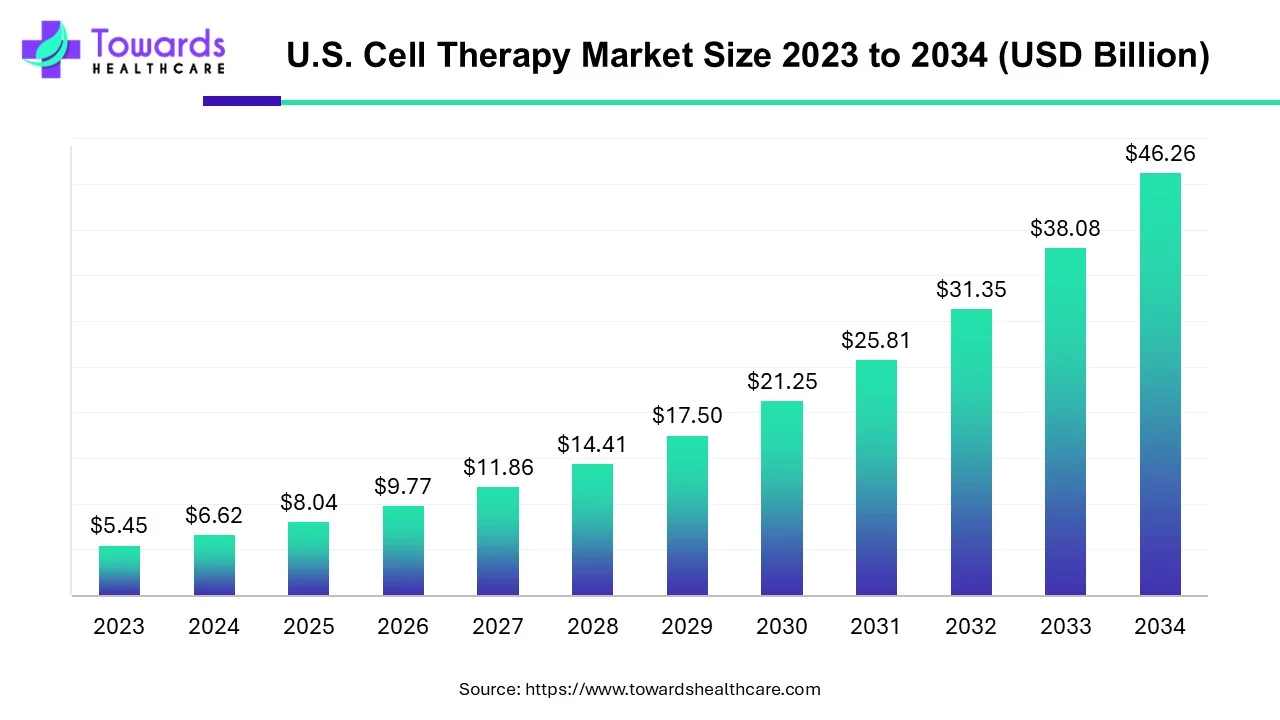
The U.S. cell therapy market size is forecast to grow at a CAGR of 21.46%, from USD 8.04 billion in 2025 to USD 46.26 billion by 2034, over the forecast period from 2025 to 2034.
Key Takeaways
- By therapy type, the autologous segment dominated the market in 2023.
- By therapy type, the allogenic therapies segment is estimated to grow at the fastest CAGR during the forecast period.
- By therapeutic area, the oncology segment dominated the market in 2023 and is expected to remain dominant during the forecast period.
Key Metrics and Overview
| Metric |
Details |
| Market Size in 2024 |
USD 6.62 Billion |
| Projected Market Size in 2034 |
USD 46.26 Billion |
| CAGR (2025 - 2034) |
21.46% |
| Market Segmentation |
By Therapy Type and By Therapeutic Area |
| Top Key Players |
Aurion Biotech, Vertex Pharmaceuticals Incorporated, Cellular Biomedicine Group, Inc, Nkarta, Inc, Atara Biotherapeutics, Inc, Johnson & Johnson, CARGO Therapeutics, Inc., Bristol-Myers Squibb Company, Selecta Bioscience, Gilead Sciences, Inc. |
Industry at a Glance
The U.S. cell therapy market encompasses research & development, therapeutics, medication, education, equipment, software, and different resources associated with cell therapy. Cell therapy is a type of treatment where a patient receives an injection, graft, or implant of live cells in order to have a therapeutic effect. It is also known as cellular therapy, cell transplantation, or cytotherapy. The most widely used and proven cell transplantation therapy is bone marrow transplants. Treatments for cancer, autoimmune illnesses, infections, urinary issues, spinal cord injuries, rebuilding damaged joint cartilage, strengthening immunocompromised people, and neurological disorders are among the potential uses of cell treatments. Hospitals, clinics, biotechnology, research & development, pharmaceutical, and educational sectors are among the main industries contributing to the growth of the U.S. cell therapy market. The future growth of the market is being aided by research and development, treatment customization, and technological improvements.
Global Cell Therapy Market Forecast
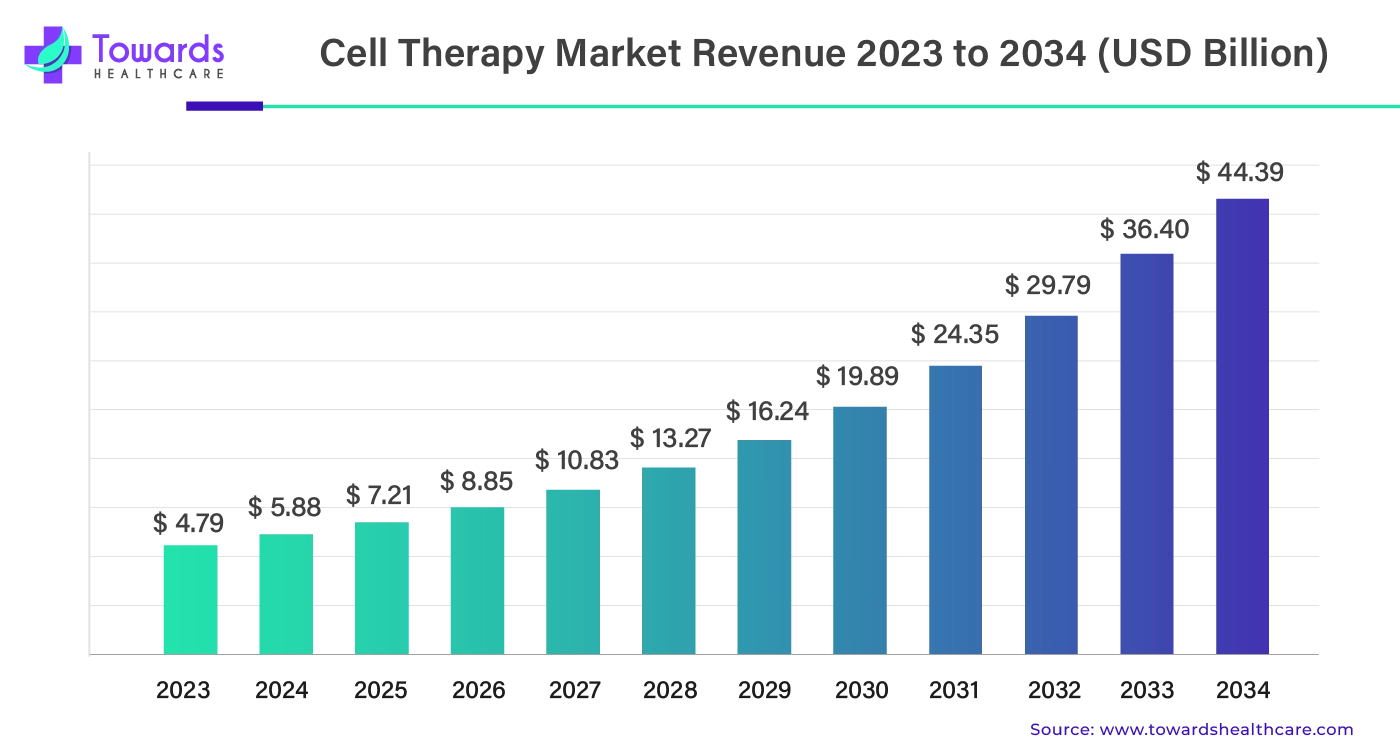
The global cell therapy market is projected to grow from an estimated US$ 7.21 billion in 2025 to US$ 44.39 billion by 2034, expanding at a compound annual growth rate (CAGR) of 22.69% between 2024 and 2034.
Cell therapy is a cutting-edge approach to treating or preventing diseases by using specially selected or modified cells outside the body. It holds exciting potential in medicine, but there are still challenges to overcome before it becomes widely available. One major hurdle is the need to improve and scale up the way these therapies are produced, which often involves automating complex manufacturing processes to meet growing clinical demand.
Over the past 20 years, research from universities and government institutions around the world has greatly expanded our understanding of how cell therapies work. Although the science is still developing, new types of cells are being studied and tested for a range of future treatments. As the field continues to evolve, cell therapy is expected to play an increasingly important role in healthcare.
Market Trends
- In April 2025, as per the 2 different clinical trials, stem cell therapies are considered a safe and effective way for the treatment of Parkinson's disease. The study used cells derived from human embryonic stem cells and human induced pluripotent stem cells, which were mentioned in the papers published in the Nature journal.
- In April 2025, lab-grown immune cells were developed by the researchers of the University of California, Irvine. It can track and clear the toxic brain buildup, which helps in restoring memory as well as brain function in mice. This was achieved by developing brain immune cells called microglia using stem cells (that developed into any type of body cell).
- In April 2025, as per the researcher from The University of Texas MD Anderson Cancer Center, significant results were obtained along with an acceptable safety profile in patients suffering from two types of advanced thyroid cancer using AIC100, which is a new chimeric antigen receptor (CAR) T cell therapy.
- In April 2025, a new CAR-T cell therapy targeting the CD30 protein (HSP-CAR30) was developed by the collaboration between the researchers from Sant Pau Research Institute (IR Sant Pau), Sant Pau Hospital, and the Josep Carreras Leukaemia Research Institute, which showed high efficacy in patients with refractory CD30+ lymphoma. According to the results of the Phase I clinical trial published in the prestigious journal Blood, stated that long-lasting responses along with enhanced clinical outcomes in treated patients are promoting the expansion of memory T cells by this new CAR-T30 therapy.
- In April 2025, the EchoBack CAR T-cell, which is a newly developed cancer-fighting immune cell, was formulated by the USC researchers. It utilizes focused ultrasound that helps to activate and sustain a powerful attack targeted at the tumors. These cells can remain effective for various days without tiring, and they only activate around the cancer cell, which in turn minimizes the damage to the healthy tissue.
Market Dynamics
The Application of Various Diseases is Driving the U.S. Cell Therapy Market’s Growth
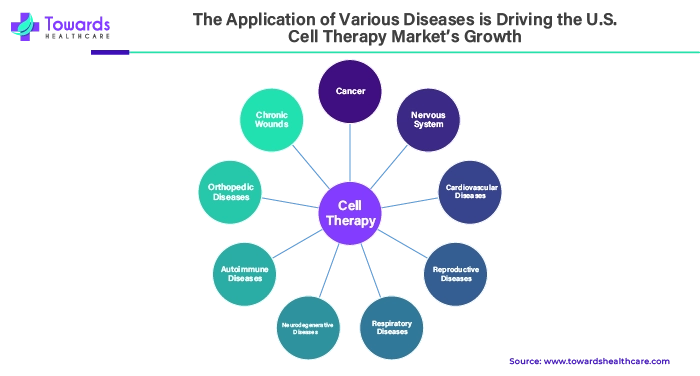
Cell therapies have applications in various diseases and are mainly used for cancer treatment. Numerous growth factors secreted by adult bone marrow stem cells allow them to develop into the type of cells needed to repair the heart and blood arteries. Stem cell treatment has the potential to promote the formation of new, healthy skin tissue, increase the synthesis of collagen, accelerate the growth of hair following cuts or hair loss, and help replace damaged or scarred tissue with newly formed, healthy tissue.
According to recent developments in the treatment of conditions like Parkinson's and Huntington's, adult stem cells injected into the brain can aid in the regeneration of new brain synapses, neurons, and cells following brain trauma or cognitive decline. For those suffering from severe and incapacitating autoimmune disorders like lupus and rheumatoid arthritis, stem cell treatment offers fresh hope. Patients suffering from pain connected to sports injuries, spinal disorders, and orthopedic issues might benefit from this therapy. As these diseases grow in the U.S., the demand for the U.S. cell therapy market is increasing.
For instance,
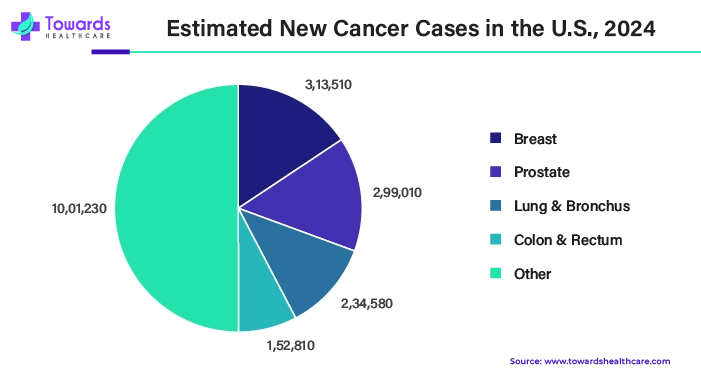
- According to the American Heart Association, a person in the U.S. passes away from CVD on average every 34 seconds. Heart disease, including heart attacks, claims the lives of around 1,905 Americans every day. Around 605,000 new cases and 200,000 recurrent cases of heart attacks occur in the U.S. annually. Recurrent or fresh strokes affect 795,000 individuals annually. 185,000 of these attacks are repeats, while about 610,000 are first strikes.
- Globally, millions of individuals are impacted by neurodegenerative disorders. An estimate from the Alzheimer's Disease Association in 2022 suggested that as many as 6.2 million Americans may be suffering from Alzheimer's. As per the Parkinson's Foundation, around one million Americans are afflicted with this condition.
- More people are suffering from autoimmune disorders. In the U.S., autoimmune diseases affect up to 50 million individuals, making it the third most common kind of illness. Women make up around 80% of those with long-term autoimmune diseases.
High Cost of Cell Therapy Restraints the Growth of the U.S. Cell Therapy Market
Among the costliest treatments available is cellular and gene therapy. Concerns regarding the affordability and accessibility of cell therapies for patients, payers, and healthcare systems both domestically and internationally are raised by their high cost—typically over $400,000 US$ and occasionally over $1 million USD per patient and technical difficulties. Additionally, the cost of cell therapy may put a strain on healthcare budgets, requiring governments and insurers to make difficult choices about how best to allocate resources and cover various products. One reason for the high cost of cell therapy is the expenses associated with its production and administration. Leukapheresis is the first stage in the multistep cell manufacturing process. Next, T cells are obtained by genetic engineering, which uses viral vectors or nonviral ways to add CAR expression. Finally, the transformed T cells are expanded in a controlled environment. Each of these procedures may need specialized tools, knowledgeable staff, and stringent quality assurance protocols.
CAR T-Cell Therapy is the Future of the U.S. Cell Therapy Market
CAR-T cell therapy is one of the newest and most promising therapies available for blood cancer. These treatments aid in the battle against cancer by using your body's immune system. When the first CAR-T cell drugs were discovered, Penn Medicine was a trailblazer. CAR T-cell therapy has a number of benefits that make it extremely promising for the treatment of cancer in the future. Certain malignancies have been effectively treated using CAR-T cells, especially blood cancers like lymphoma and leukemia. CAR T cells are designed to target a particular antigen present in cancerous cells. This customization lessens adverse effects and minimizes damage to healthy tissues. CAR T cells are designed to proliferate and endure inside the body. This implies that they may provide sustained defense against the return of cancer.
For instance,
- In June 2024, in order to enable pharmaceutical companies and bigger healthcare providers to produce chimeric antigen receptor T (CAR T) cell therapies and other cell-based treatments more quickly and affordably, Cytiva introduced its Sefia next-generation manufacturing platform. In order to create Sefia, Kite, a Gilead firm, collaborated with Cytiva utilizing both companies' R&D and manufacturing capabilities as well as Kite's experience developing two popular CAR T treatments, Tecartus® (brexucabtagene autoleucel) and Yescarta® (axicabtagene ciloleucel).
Segmentations Highlights
Why did the Autologous Segment Dominate the U.S. Cell Therapy Market?
By therapy type, the autologous segment dominated the U.S. cell therapy market in 2023. The first step in autologous cell treatment is usually to remove tissues or cells from the patient, such as bone marrow or blood. Autologous cell treatments include autologous skin grafting, autologous CAR T cell therapies, and platelet-rich plasma therapy. Autologous methods have the important benefit of reducing the danger of immunological rejection in patients and fostering long-term recovery. There are fewer adverse effects and a greater likelihood of success when the risk profile is favorable. Additionally, patients receiving autologous cell treatments have access to customized therapy choices, which enables more focused and efficient interventions. Cardiovascular disorders can be effectively treated using autologous cell treatments, cancer, neurodegenerative illnesses and orthopedic problems.
For instance,
- In March 2024, the Blood and Marrow Transplantation (BMT) and Cell Therapy Program at City of Hope Cancer Center Atlanta was launched, according to a statement from City of Hope®, one of the biggest cancer research and treatment organizations in the U.S.. One of the biggest and most effective transplant programs in the U.S., the City of Hope was a pioneer in BMT and has performed close to 19,000 successful transplants since the program's founding in 1976. This spring, the City of Hope Atlanta will perform its first autologous stem cell transplant surgery and offer BiTE treatment.
By therapy type, the allogenic therapies segment is estimated to grow at the fastest CAGR during the forecast period. Various advantages of allogenic cell therapies are boosting the growth of the segment in the U.S. cell therapy market. The fact that there are several possible cell sources for allogeneic cell therapy, such as donated tissues, bone marrow, induced pluripotent stem cell and embryonic stem cells, placenta, and umbilical cord blood, is advantageous. Patients with Sezary syndrome, myelodysplastic syndrome, acute myeloid leukemia, and cutaneous T cell lymphoma may benefit from allogeneic stem cell transplantation (Allo-SCT).
Convenient regeneration therapeutics are drawing interest in allogeneic adult stem cells. They provide an answer to resistive barriers in allogeneic therapies because they may be designed to elude the recipient's immune system. Mesenchymal stem cell treatment, hematopoietic stem cell transplantation, induced pluripotent stem cell therapy, and chimeric antigen receptor T cell therapy are a few examples of allogeneic cell therapies.
For instance,
- In April 2024, in the U.S., VetStem, Inc. introduced the first allogeneic off-the-shelf cell medication product to be evaluated by the FDA. This leucoreduced, allogeneic, pooled, freeze-dried PRP is meant to offer a concentrated platelet in plasma source unique to canines for intra-articular injection. The horse version is expected to pass FDA scrutiny early in May and go on sale the same month.
What Made the Oncology Segment Dominant in the U.S. Cell Therapy Market?
By therapeutic area, the oncology segment dominated the U.S. cell therapy market in 2023 and is expected to remain dominant during the forecast period. A promising new treatment in the battle against cancer is stem cell treatment, which encompasses all operations involving stem cells. Because it improves the target of tumors, it may increase the therapeutic effectiveness of other medicines while lowering off-target occurrences. Preclinical trials have examined a wide range of stem cell-based approaches, demonstrating both considerable promise and difficulties in the treatment of cancer.
Stem cell therapy has been used to produce a number of cancer treatment options, such as HSC transplantation, MSC infusion for post-cancer treatment, stem cells as therapeutic carriers, the creation of vaccines, and the development of immune effector cells. Numerous blood malignancies have already received approval for cell treatments, and it is believed that many other tumors may benefit as well. Cell treatments can be designed to target and eliminate tumor cells that have certain genetic abnormalities, sparing healthy cells from harm.
For instance,
- In February 2022, after four prior treatments, the FDA approved J&J and Legend's CAR-T therapy Carvykti to treat multiple myeloma. The medication, previously known as cilta-cel, now has a fair chance to compete with Bristol and Bluebird Bio spinout 2seventy Bio's BCMA-targeted CAR-T medication Abecma, which was introduced a year ago as a fifth-line therapy. Thanks to the label.
Latest Announcements by Industry Leaders
In August 2025, after announcing a $100,000 G-Rex® Grant, the Research Investigator at Sidra Medicine, M.D., Ph.D. Sara Deola commented that innovations in precision medicine are the main focus of Sidra Medicine, which is a pioneering healthcare institution. For pediatric diseases like cancers, rare and genetic diseases, this GMP facility provides better treatment protocols. Thus, with this grant, the protocols will be accelerated, specifically accelerating the pediatric oncology program of Sidra Medicine. Moreover, the G-Rex® method is considered to be outstanding, as it will provide a cell manufacturing platform suitable for T cell phenotype, promoting pediatric patient care and cure.
The U.S. Cell Therapy Market Top Companies
Read further to see how top players are redefining the U.S. Cell Therapy Market: https://www.towardshealthcare.com/companies/us-cell-therapy-companies
Atara Biotherapeutics Inc. Pipeline for Cell Therapy
| Company Name |
Atara Biotherapeutics Inc. |
| Headquarters |
California, U.S. |
| Pipeline |
In order to set the path for future therapies, the firm is making a lot of effort to address important unmet needs in autoimmune disorders and cancer. Numerous cell treatments are being researched.
- Tab-cel® is in the early stages of research and is being studied for individuals with Epstein-Barr virus-associated post-transplant lymphoproliferative disorder (EBV+ PTLD). Targeting and eradicating EBV-infected cells is the goal of Tab-cel, an allogeneic T-cell immunotherapy.
- The EBV T-cell platform is used in the clinical development of ATA3219, an allogeneic anti-CD19 chimeric antigen receptor (CAR) T-cell treatment with a memory phenotype, unedited T-cell receptor, and next-generation 1XX co-stimulatory domain.
- Aiming for an IND application in H2 2025, the off-the-shelf, allogeneic CD19/CD20 CAR T program is ATA3431.
- EBV-infected B cells and plasma cells, which are thought to be the etiology of MS, are the focus of the experimental allogeneic T-cell immunotherapy known as ATA188.
|
Gilead Sciences Inc. Pipeline for Cell Therapy
| Company Name |
Gilead Sciences Inc. |
| Headquarters |
California, U.S. |
| Pipeline |
List of cell therapies under development:
- CD19 CAR (KITE-197), Relapsed/refractory diffuse large B-cell lymphoma (Phase 1)
- CD19/CD20 bicistronic (KITE-753), Relapsed/refractory diffuse large B-cell lymphoma (Phase 1)
- CD19/CD20 bicistronic (KITE-363), Relapsed/refractory diffuse large B-cell lymphoma (Phase 1)
- Anitocabtagene autoleucel (iMMagine-1), Relapsed/refractory multiple myeloma (Phase 2)
- Brexucabtagene autoleucel (ZUMA-4), Pediatric acute lymphocytic leukemia/ non-Hodgkin lymphoma (Phase 2)
- Axicabtagene ciloleucel (ZUMA-24), 2L large B-cell lymphoma outpatient (Phase 2)
- Axicabtagene ciloleucel (ZUMA-23), 1L high-risk large B-cell lymphoma (Phase 3)
- Axicabtagene ciloleucel (ZUMA-22), 2L+ high-risk follicular lymphoma (Phase 3)
|
Company Revenue
- In July 2025, Johnson & Johnson released their revenue reports, which showed adjusted earnings of $2.77 per share for the second quarter of 2025, with 1.8% year-over-year growth. Furthermore, a growth in sales was observed with $23.74 billion compared to the consensus of $22.85 billion, up to 5.8% year over year. Additionally, the operational growth and adjusted operational growth were noted to be 4.6% and 3%, respectively. Moreover, a rise of 4.9% or 3.8% operationally to $15.20 billion was reported by the sales of innovative medicines.
- In March 2025, the fourth quarter financial results and year ended for December 31, 2024, were released by Nkarta, Inc., which is a clinical-stage biopharmaceutical company developing engineered natural killer (NK) cell therapies. The marketable securities as of 31st December 2024, including investments, cash, cash equivalents, and restricted cash, were noted to be $380.5 million.
- $23.1 million and $96.7 million were the R&D expenses reported for the fourth quarter of 2024 and for the full year 2024, respectively. Similarly, the general and administrative (G&A) expenses were noted to be $7.8 million and $31.5 million for the fourth quarter of 2024 and the full year 2024, respectively.
- In February 2025, the fourth quarter financial results and year ended for December 31, 2024, were released by Vertex, Inc. The total revenues showed a rise of 15.2% year-over-year increase with $178.5 million for Q4 and a 16.5% increase to $666.8 million for the full year.
- Strong operational cash flow was achieved with an adjusted EBITDA of $38.1 million and non-GAAP net income of $25.5 million. While 16.8% rise for Q4 and 27.0% for the full year were reported, highlighting the significant growth in software subscriptions and cloud revenues. Moreover, revenue growth between $760 million and $768 million will be the goal of the company for 2025.
Recent Developments
- In April 2024, As the firm increases its core pharmacy business, Walgreens is investing in its capabilities and extending its services for specialty pharmacies in an effort to enhance patient outcomes and give payers and partners more value. Today, the firm unveiled Walgreens Specialty Pharmacy, a comprehensive service that increases patients' access to care for chronic, complicated illnesses and facilitates collaborations that boost the profitability of Walgreens' pharmacy division. Additionally, the business is investing in areas that will change the services it offers as a specialist pharmacy, such as gene and cell therapy.
- In March 2024, for children under the age of eighteen who have the most prevalent kind of lupus, Seattle Children's has been given permission by the FDA to begin the nation's first clinical trial with chimeric antigen receptor (CAR) T cells. Launching this first-in-country experiment might provide patients with treatment that could change their lives, which is why Seattle Children's is delighted.
- In November 2022, the UC San Diego Alpha Stem Cell Clinic received $8 million from the California Institute for Regenerative Medicine (CIRM) as part of a state-wide initiative to improve stem cell therapy. The money will help the clinic fulfill its goal of providing patients with illnesses that are challenging to cure with novel stem cell-based treatments.
Segment Covered in U.S. Cell Therapy Market Report
By Therapy Type
- Allogeneic Therapies
- Stem Cell Therapies
- Hematopoietic Stem Cell Therapies
- Mesenchymal Stem Cell Therapies
- Non-Stem Cell Therapies
- Keratinocytes & Fibroblast-based Therapies
- Others
- Autologous Therapies
- T-Cell Therapies
- CAR T Cell Therapy
- T Cell Receptor (TCR)-based
- Others
By Therapeutic Area
- Oncology
- Dermatology
- Others





