January 2026

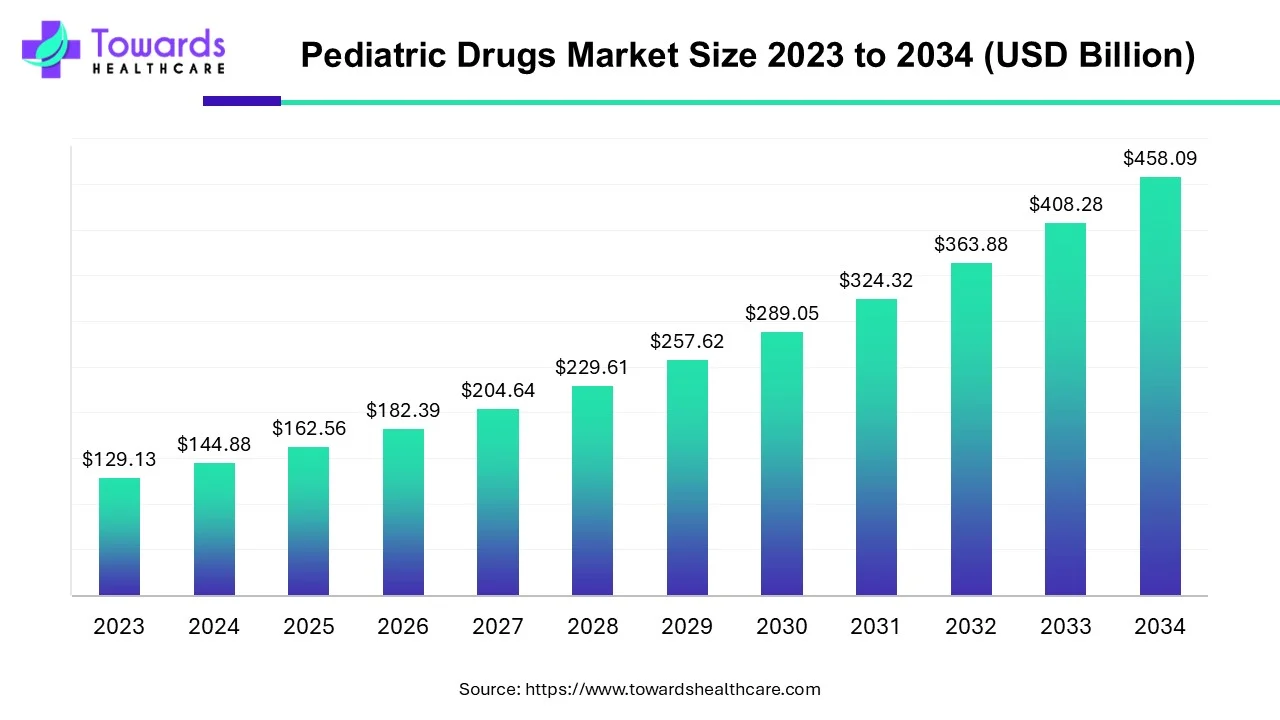
The global pediatric drugs market size is forecasted to expand from USD 162.56 billion in 2025 to USD 458.09 billion by 2034, growing at a CAGR of 12.2% from 2025 to 2034, as a result of the increasing prevalence of pediatric disorders, and rising pediatric population.
In March 2023, WHO released the first list of priority paediatric antibiotic compositions, enabling more concentrated research and development activities that tackle the specific requirements of infants and children.
Artificial intelligence (AI) has the potential to disrupt the healthcare sector by driving the latest innovations to enhance patient care. It can be used to develop novel drugs and identify novel drug targets related to a particular disease, enabling researchers to develop effective drugs. It can suggest personalized medicines based on the patient’s condition. AI enhances the accuracy and precision of pediatric medications, increasing medication adherence. It can also facilitate pharmacists and physicians in precise dose calculations by analyzing a patient’s weight and age. Moreover, AI can streamline the entire clinical trial process involving pediatrics, optimizing trial design. This leads to more efficient protocols and improved decision-making.
Pharmaceutical companies are now working more on making medicines specifically for paediatrics, considering age, weight, and how their bodies develop. This is because children have unique traits that can affect how medicines work for them. The market for these pediatric drugs is growing because more kids are getting sick, and there are better ways to design drugs. But there are challenges, like figuring out the right amount of medicine for different ages and dealing with smaller groups of patients, which can make the drugs more expensive. Organizations like the European Medical Agency (EMA) and the Food and Drug Administration (FDA) are making rules to encourage research and ensure that medicines for children are safe and work well.
The market for pediatric drugs is growing because kids often face health issues like stomach, allergy, and respiratory problems due to their weaker immune systems. This market includes drugs for conditions like breathing disorders, autoimmune diseases, stomach issues, heart-related problems, and others. Medicines can be taken orally, applied on the skin, injected, or through other ways. This market spans North America, Europe, Asia-Pacific, the Middle East, Africa, and Latin America, with specific data for 17 countries.
Individuals aged 2 to 12 receive pharmaceutical interventions specifically designed for pediatric use. The approach to pediatric therapy is fundamentally different from that of adults, primarily stemming from the imperative to tailor dosage regimens to the unique requirements of pediatric patients. Additionally, differentiation is imperative because the physiological response of a child to a given drug may diverge significantly from that observed in an adult population.
Thus, the distinct nature of pediatric therapy is essential for ensuring optimal efficacy and safety in this specific age group. Furthermore, the market experiences a positive impetus due to the expanding landscape of clinical trials aimed at introducing novel pediatric pharmaceutical products. This multifaceted synergy of elements substantiates the robust growth trajectory observed in the pediatric drugs market.
The following factors propel the pediatric drugs market growth
The expansion of the pediatric drugs market is driven by several factors, such as advancements in pediatric research, the broadening range of clinical trials, and increased investment in developing medications personalised for children. This trend creates substantial business prospects for pharmaceutical and biotechnology companies specialising in pediatric drug formulations. For instance, in August 2023, Aurobindo Pharma Ltd announced the release of their HIV triple combination medicine to pediatric patients in around 123 low- and middle-income countries.
Additionally, companies offering contract manufacturing services for pediatric pharmaceuticals stand to benefit. Collaborative research ventures, innovative drug delivery methods, and strategic partnerships with healthcare providers further facilitate market growth. Overall, these developments present promising opportunities for growth in the pediatric drugs market.
| Approval Date | Drug Approved for Childhood Cancer | Indication |
| March, 2023 | Debrafenib | Trametinib , when used together, is approved for child aged 1 and older to treat Low grade Glioma (Brain Tumor) |
| February, 2023 | Nivolumab | This drug is given to Adults and children aged 12 and above with Melanoma. In addition, treatment is recommended to help prevent the cancer from coming back. |
| July, 2022 | Crizotinib | Treatment of adult and pediatric patients 1 year of age and older with Refractory inflammatory myofibroblastic tumor (IMT) |
| June, 2022 | Trametinib | For Adults and Pediatric Patient aged 12 and older with melanoma and lymph node involvement or metastatic disease post complete resection, adjuvant treatment is recommended |
The wider utilization of off-label drugs in the pediatric population underscores the need for specialised pharmaceuticals customised to pediatric drug requirements, fueling innovation and expansion in the pediatric drug market. The expansion of the pediatric drug market is primarily driven by the increasing prevalence of health issues in children, including obesity, diabetes, asthma, and neurological disorders.
This has led to a rising demand for specialised pharmaceuticals personalised to the unique needs of the pediatric population. Recognising children's distinct physiological and developmental characteristics, the pharmaceutical industry increasingly focuses on research and developing drugs specifically designed for them.
The heightened awareness among parents, caregivers, and healthcare practitioners regarding the importance of addressing children's healthcare needs has fueled the demand for pediatric medications. This advocacy has prompted governments and regulatory bodies to introduce incentives, such as market exclusivity and extended patent protection for pediatric drugs, encouraging research in this field. Consequently, these initiatives have attracted pharmaceutical investments and fostered innovation. The surge in pediatric health issues is propelling the growth of the pediatric drug market, underscoring its crucial role in safeguarding and improving the well-being of children globally.
The pediatric drugs market is anticipated to be predominantly influenced by the respiratory disorder drugs segment, which holds the largest market share. This is primarily attributed to factors such as compromised immunity, heightened pollution levels, and increased exposure to various allergens, leading to chronic respiratory disorders like Chronic Obstructive Pulmonary Disease (COPD). COPD, a significant contributor to the global pediatric healthcare burden, has seen a surge in demand for effective treatments.
The segment’s growth is driven by the escalating prevalence of rare autoimmune disorders linked to various cancers, genetic disorders, and other autoimmune conditions. The market is further propelled by initiatives to develop drugs for rare diseases, exemplified by the FDA's approval of RUZURGI (amifampridine) tablets from Jacobus Pharmaceutical Company in May 2019. These prescription drugs, though associated with high costs, are contributing to the rapid growth of the global pediatric drugs market.
The variance between the high rate of childhood illness, roughly 60%, and the narrow focus of pharmacological trials, about 12%, highlights the unrealized potential in the pediatric medication market. This disparity, coupled with a greater understanding of the particular healthcare requirements of children, has spurred more focus and funding for pediatric medication research. The growth of the pediatric medication business has also been aided by regulatory incentives, including pediatric exclusivity and an understanding of the moral need to address children's health. As a result, pharmaceutical companies are working harder on research and development projects to close this gap and satisfy the unique therapeutic needs of the diseases. They are mostly prevalent with childhood cancer in North America.
According to the Nationwide Children’s Hospital’s May 2023 estimates, in the U.S., around 90% of 17,000 paediatrics are diagnosed with cancer every year. 1,000+ Medical research grants at 150 institutions, 240+ Ongoing clinical trials funded each year. The increasing incidence of chronic disorders, encompassing conditions such as anorexia, asthma, congenital disabilities, growth deficiencies, diabetes, childhood cancer, juvenile diabetes, and attention deficit hyperactivity, is projected to positively impact the growth trajectory of the market. This surge in chronic disorders is a primary factor contributing to heightened demand for pediatric drugs.
Obesity is one of the most prevalent conditions among children. Obesity is a major problem that leads to several diseases like Type 2 Diabetes, Hypertension, Hyperlipidemia, Liver and kidney diseases, Hypertension, Cancer Risk and others.
The escalation of healthcare investment plays a pivotal role in shaping the trajectory of the pediatric drug market. A substantial determinant contributing to market growth is the surge in healthcare expenditure, facilitating the enhancement of healthcare infrastructure. Notably, government bodies are proactively augmenting this development by allocating increased funding to bolster healthcare facilities, exerting a profound impact on market dynamics.
Moreover, the concerted efforts of both public and private entities to raise awareness, coupled with a notable uptick in childhood obesity cases, are poised to further propel the pediatric drugs market. This phenomenon is further accentuated by evolving lifestyle patterns and a burgeoning population, collectively fostering the expansive growth of the pediatric drugs sector. The convergence of these factors delineates a landscape where healthcare investments, governmental initiatives, and societal changes converge to drive advancements in pediatric pharmaceuticals.
The abbreviated duration of market exclusivity poses a significant obstacle to the growth of the pediatric drugs market. In contrast to pharmaceuticals customised for adults, pediatric medications often face shorter market exclusivity and patent protection periods. This diminishes the financial allure for pharmaceutical companies to invest in pediatric drug development, as they are inclined towards markets where research and development investments can be recouped with substantial profits. The pediatric sector encounters challenges due to smaller patient populations and limited exclusivity periods, making it more difficult to justify allocating resources for research and development. This limitation mainly affects the development of drugs for rare pediatric diseases, where patient numbers are exceptionally restricted.
Consequently, a considerable number of children resort to off-label use of medications intended for adults, posing potential risks to safety and effectiveness. Addressing this constraint necessitates policy and regulatory reforms that offer enhanced incentives for pediatric drug development, ensuring children can access secure and efficacious treatments.
The paediatrics drugs market is categorized by type, encompassing analgesic and antipyretic, central nervous system drugs, especially antibiotics, gastrointestinal drugs, respiratory drugs, and vaccines. The respiratory drugs segment held a dominant presence in 2024, driven by children's lower immunity, increasing pollution, and allergen exposure leading to chronic respiratory disorders, including Chronic obstructive pulmonary disease. Montelukast is notably employed for asthma prevention in children 2 years and older, particularly for exercise-induced bronchoconstriction.
By type, the gastrointestinal drugs segment is predicted to witness significant growth in the market over the forecast period. Some common gastrointestinal disorders in pediatrics include inflammatory bowel disease (IBD), constipation, diarrhea, gastroesophageal reflux, and abdominal pain. The rising incidences of GI disorders such as genetic disorders, food allergies, and structural abnormalities boost the segment’s growth. Ursodiol, clarithromycin, mesalamine, and budesonide are some drugs prescribed to pediatrics. The availability of child-friendly formulations and natural ailments also promote the segment’s growth.
The Pediatrics Medicine Market is categorized based on the route of administration, namely topical, oral, parental, and others. The Oral segment dominated with the highest revenue share. This is attributed to the preference for non-invasive oral delivery in pediatric patients, minimizing pain and enhancing parental comfort, resulting in improved medication compliance.
This growth is driven by the minimal risk of adverse reactions and drug interactions, along with the ease of administration to children. Additionally, factors such as cost-effectiveness, lower drug quantities, and the concentrated application of antibiotics in affected areas contribute to the growth of this route of administration.
By route of administration, the parenteral segment is projected to expand rapidly in the market in the coming years. The parenteral route is most widely preferred for pediatrics, as very small children are unable to take medications through the oral route. The inability of pediatrics to absorb and metabolize drugs in the GI tract increases the need for the parenteral route. Additionally, medications delivered through the parenteral route are absorbed more quickly and present a rapid onset of action. Most of the vaccines are administered through the parenteral route to achieve desired outcomes. Hence, rising immunization programs also propel the segment’s growth.
By distribution channel, the hospital pharmacies segment led the global market in 2024. Hospital pharmacies can contribute to pediatric drug market growth by ensuring a consistent supply of specialized medications for young patients, collaborating with healthcare providers to optimize treatment plans, and counselling parents on medication administration. They have suitable infrastructure for storing and dispensing pediatric medications as well as follow stringent regulatory guidelines.
By distribution channel, the online pharmacies segment is anticipated to grow with the highest CAGR in the market during the studied years. Online pharmacies enhance accessibility, especially for parents in remote areas. They can provide a wide range of pediatric medications, offer educational resources, and support telemedicine initiatives, making it easier for parents to consult healthcare professionals about their children's health.
North America dominates the pediatric drugs market due to rising healthcare spending, supportive regulatory framework, and quick adoption of advanced technologies across the U.S. and Canada. The growth in this region is primarily attributed to its robust healthcare infrastructure, substantial research and development capabilities, and a significant pediatric patient demographic. The region's dominance is further bolstered by favourable regulatory frameworks and government incentives, fostering the development and accessibility of pediatric medications.
The market benefits from North America's dedicated focus on pediatric healthcare heightened awareness among parents and healthcare professionals, and the region's economic strength. Additionally, the presence of leading pharmaceutical companies further solidifies North America's prominent position in the global pediatric drugs market.
The U.S. market is growing due to rising childhood health issues, increased awareness and early treatment, supportive FDA regulations, scientific advancements, and improved insurance coverage, all driving demand for specialized, effective pediatric medications.
Canada’s market is expanding due to a rise in childhood chronic diseases, government initiatives like Health Canada’s Pediatric Drug Action Plan, and growing research in child-specific medicines. Increased public awareness and advocacy are also driving demand for safer, more effective pediatric treatments across the country.
During the forecast period, the Asia-Pacific region is poised for significant pediatric drug market growth. This expansion is driven by various vital factors, including the region's substantial and expanding pediatric population, leading to an increased demand for specialized healthcare solutions for children. The economic prosperity and expanding middle-class demographic in Asia-Pacific also enhance affordability and accessibility to pediatric medications.
Government initiatives and increased regional healthcare investments are pivotal in fostering market growth. The pharmaceutical industry in Asia-Pacific is channelling more resources towards developing medications tailored for pediatric patients. This concerted effort has positioned the region as a significant influencer in the pediatric drugs market, emerging as a central hub for research, development, and distribution of pharmaceuticals for children.
China's market is accelerating due to government reforms like priority reviews and fast-track approvals, increased pediatric clinical trials, and a focus on child-friendly formulations. These initiatives aim to address the shortage of pediatric medications and improve healthcare outcomes for children.
A significant portion of the population, approximately 26%, comprises children aged 0–16 years, creating substantial demand for pediatric healthcare services. Government initiatives like the National Health Mission and Rashtriya Bal Swasthya Karyakram focus on improving child health outcomes. Additionally, the Pradhan Mantri Bharatiya Janaushadhi Pariyojana enhances access to affordable generic medicines. The growing awareness among parents and the development of pediatric-specific formulations further contribute to the market's growth.
Europe's pediatric drug market is expanding significantly due to the 2007 EU Paediatric Regulation, which mandates Paediatric Investigation Plans (PIPs) for new medicines. This framework has led to over 290 new pediatric medicines and a 50% increase in child-inclusive clinical trials between 2007 and 2016, enhancing treatment options for children across Europe.
The UK's market is expanding due to integrated healthcare reforms, increased NHS investment in children's mental health, and a growing focus on personalized treatments. Rising rates of chronic pediatric conditions and advancements in telehealth and digital infrastructure further drive demand for specialized, child-friendly medications.
France’s market is expanding due to rising childhood chronic diseases, strong government investment in biomedical innovation, and early access programs like ATU. Enhanced pediatric healthcare infrastructure, supportive regulations, and increasing demand for child-specific treatments are driving growth and ensuring better health outcomes for the French pediatric population.
Jeffrey M. Dayno, President and CEO of Harmony Biosciences, commented on the FDA approval of WAKIX for excessive daytime sleepiness (EDS), that EDS is the primary symptom experienced by all patients with narcolepsy. The product is the first-and-only FDA-approved non-scheduled treatment option for narcolepsy and is an essential treatment option available to pediatric patients 6 years and older.
By Drug Type
By Route of Administration
By Distribution Channel
By Geography
January 2026
January 2026
January 2026
January 2026