February 2026

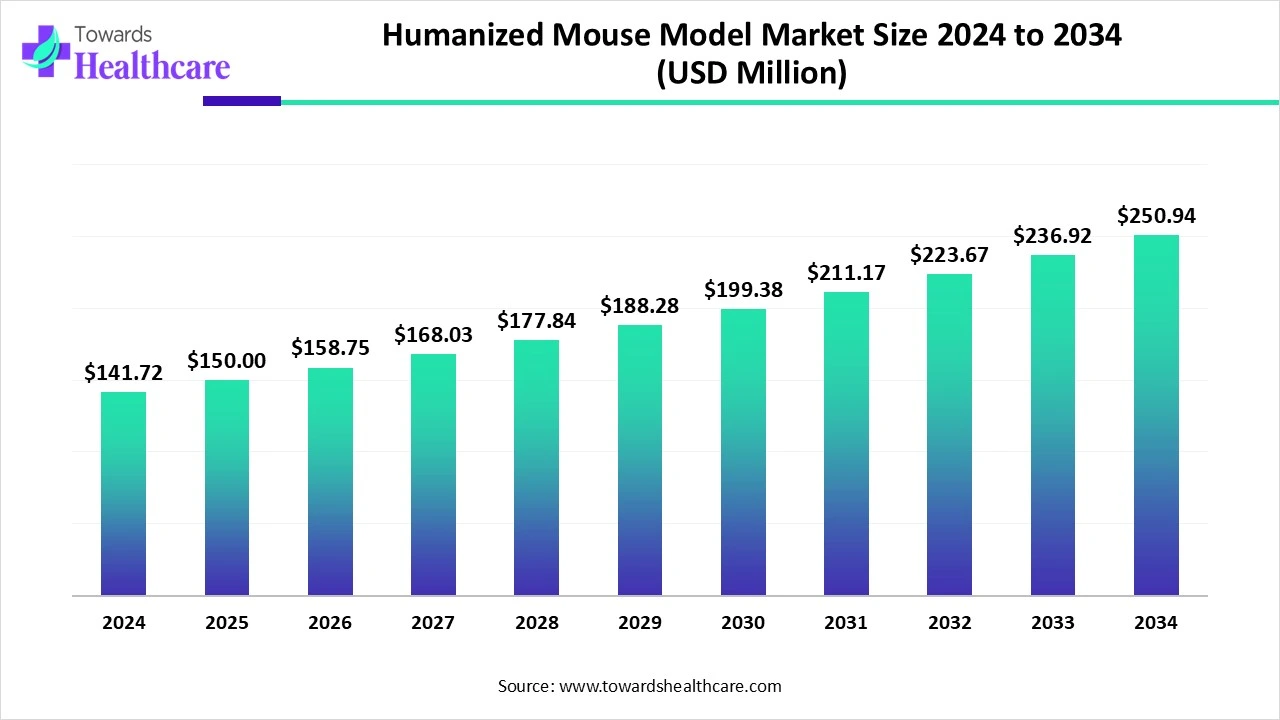
The global humanized mouse model market size is calculated at US$ 141.72 in 2024, grew to US$ 150 million in 2025, and is projected to reach around US$ 250.94 million by 2034. The market is expanding at a CAGR of 5.84% between 2025 and 2034.
The humanized mouse model has emerged as a powerful tool in the quickly evolving fields of biomedical research and pharmaceutical innovation, aiding in the transition from in vitro to clinical trials. Designed to include functional human genes, cells, or tissues, these models offer unprecedented insight into complex human biological systems. As the demand for translationally relevant preclinical models grows, company executives are turning to humanized mice to improve their approach to drug development, immunology, infectious disease research, and other areas.
| Metric | Details |
| Market Size in 2025 | USD 150 million |
| Projected Market Size in 2034 | USD 250.94 million |
| CAGR (2025 - 2034) | 5.84% |
| Leading Region | North America |
| Market Segmentation | By Model Type, By Application, By End User, By Region |
| Top Key Players | HuMurine Technologies, Trans Genic Inc., Champions Oncology Inc., Genoway S.A., Crown Bioscience Inc., Axenis S.A.S, Taconic Biosciences Inc., Horizon Discovery Group PLC, Ingenious Targeting Laboratory, The Jackson Laboratory, Vitalstar Biotechnology Co. Ltd., Altogen Labs, Aragen Life Sciences Pvt. Ltd., Biocytogen Pharmaceuticals Co., Ltd., BioSafety Research Center Inc., Charles River Laboratories International Inc., Creative Biolabs, Cyagen Biosciences, Gempharmatech Co. Ltd., HBM Holdings Ltd., Hera Biolabs Inc., Inotiv Inc., JANVIER LABS, JSR Corp., Oncodesign Precision Medicine, Ozgene Pty Ltd., PerkinElmer Inc., Pharmatest services |
An animal that has had a gene from a human being inserted into it is called a "humanized mouse." These might be human tissue fragments, human tumors, humanized immune systems, short snippets of human DNA, or elements of the human microbiome. These animal models can be used for preclinical in vivo studies of human diseases. Humanized mouse models have been widely used in a number of research areas, including cancer, infectious diseases, and acquired immunodeficiency syndrome.
In January 2025, a strategic partnership was launched between Preclina Inc., a preclinical contract research firm that specializes in immune-related illnesses and humanized animal models, and ABC Biopply AG, a pioneer inventor of humanized multi-organoid disease models. (Source - Swissbiotech)
An important step in transforming genetic research is the development of algorithms that use artificial intelligence (AI) to analyze the vast volumes of genomic data in a much more accurate and efficient manner. AI has been applied to biotechnology and genetic engineering to improve accuracy, expedite research, and provide new opportunities. The result of these interdisciplinary disciplines collaborating is the emergence of new sciences such as systems biology and synthetic biology. Tissue engineering is enhanced by artificial intelligence (AI) through cell culture optimization, biomaterial design, computational modeling, and customized treatment.
How does Personalized Medicine Promote the Humanized Mouse Model Market?
Personalized medicine techniques benefit from the use of humanized animal models. Humanized animal models are distinct and appropriate for testing drugs and other therapeutics since they are made from a person's cells, tissue, or DNA. Humanized mouse models are still crucial for the pre-clinical development of personalized medicine techniques because they yield data that will guide the planning of clinical trials in patients with respect to the optimal dosage, level of functional protein expression requirements, dosage schedule, mode of administration, etc. The ultimate goal is to provide patients with drugs that are particularly tailored to their needs in order to enhance their overall health results.
The Small Size of the Animal Model is a Drawback
One of the main obstacles to using humanized mice is their small size. The amount of blood and the number of cells that can be extracted from tissues are lower than in larger animal models or sick humans.
Development of a Humanized Mouse Model for Rare Disease
Humanized mouse models are being developed in response to the need for better research tools for rare and complex diseases. Humanized models that can recreate certain sickness traits and symptoms allow researchers to examine rare diseases that are sometimes difficult to simulate. This approach promotes market expansion by broadening the scope of illnesses that may be studied and managed using precision drugs.
By model type, the genetic segment held the largest revenue of the humanized mouse model market in 2024. It is possible to express human genes or proteins in humanized animal models by utilizing genetic engineering techniques to integrate human genes into the animal genome. These models are used to recreate the mechanisms and progression of real diseases, investigate gene function, and evaluate the efficacy and safety of therapeutic drugs.
By model type, the cell-based (CD34, PBMC, and BLT) segment is expected to grow at the fastest CAGR in the humanized mouse model market during the forecast period. The BLT mouse model, commonly used to research the human immune system, is arguably the most reliable method for examining the relationship between HIV and the human immune system. The BLT mouse model, commonly used to research the human immune system, is arguably the most reliable method for examining the relationship between HIV and the human immune system. Compounds for infectious illnesses and graft rejection are investigated and evaluated in vivo using humanized PBMC mice.
By application, the oncology segment dominated the humanized mouse model market in 2024. Globally, the number of cancer cases is expected to rise from 20 million in 2022 to 35.3 million by 2050, a 76.6% increase. Both new and known immunotherapeutic drugs may be tested for effectiveness using humanized mouse models, which are particularly useful for researching combination therapy approaches. The most realistic platform for researching and simulating many facets of cancer immunology and immunotherapy has been made available by humanized mouse models.
By application, the infectious diseases segment is expected to grow at the fastest CAGR in the humanized mouse model market during the forecast period. According to a comprehensive global assessment of antibiotic resistance, about 39 million people will die from antibiotic-resistant illnesses between now and 2050. Plasmodium species, which can cause hepatitis, malaria, or HIV-1, are estimated by the World Health Organization to infect over 600 million people worldwide and kill more than 5 million people annually.
By end-user, the pharmaceutical & biotechnology companies segment held the largest revenue of the humanized mouse model market in 2024. Biotechnology and pharmaceutical companies are both involved in the development of new drugs and healthcare products. These products require continuous testing before getting approved for commercial launched. Hence, humanized mouse models play a crucial role during the clinical trials in drug development and testing. Humanized models increase the chances of a drug's efficacy, and also hold potential for companies in developing personalized medicines.
By end-user, the contract research organizations segment is expected to grow at the fastest CAGR in the humanized mouse model market during the forecast period. CROs are playing a crucial role in drug development and clinical trial procedures. Both the biotechnology and pharmaceutical industries use third-party services for services, including supply chain management, transportation, etc. CROs are highly specialized and hence provide high-quality services that increase the efficiency, reduce costs, and improve the overall flow of work, ultimately leading to enhanced drug development.
North America dominated the humanized mouse model market in 2024. The main factors driving this regional market's growth are growing biomedical research, pharmaceutical R&D and CRO preclinical activities, animal care organizations ensuring continued and responsible animal use, growing monoclonal antibody production in the U.S., along with growing stem cell research, and federal funding for protein drug development in Canada. Growth in the regional market is also being driven by the demand for early detection of cardiovascular and malignant illnesses, as well as a growing number of R&D projects.
The U.S. led the market due to the growing number of clinical trials. Clinical studies in the U.S. need a rigorous and regulated process to ensure the safety and efficacy of new medical therapies or interventions. Because of the U.S.'s robust research infrastructure, diverse patient pool, stringent regulatory requirements, and extensive corporate relationships, the majority of clinical trials are conducted there. The U.S. is a global leader in clinical research that increases medical knowledge because of its unparalleled advantages, and sponsors and researchers regularly choose it as their preferred location.
Canada is fourth in terms of clinical trial sites, accounts for 4% of clinical trials globally, and tops the G7 in clinical trial productivity (number of trials per population). In 2023, the country ranked fourth globally in terms of ongoing research and third globally in terms of new clinical trials. More than 40 organizations of academic healthcare groups, 17 medical institutions, and more than 15,000 researchers call Canada home.
Asia Pacific is estimated to host the fastest-growing humanized mouse model market during the forecast period, due to substantial developments in biomedical research and higher expenditures on healthcare facilities. The region's thriving biotechnology and pharmaceutical industries, together with a rising focus on personalized treatment, are driving this rapid rise. The biotechnology and pharmaceutical industries in Asia Pacific have grown quickly.
China, which is already making rapid progress in biotechnology, wants to speed up the creation of new drugs by cutting down on the amount of time it takes for authorities to assess clinical studies. China's National Medical Products Administration (NMPA) is recommending reducing the clinical trial review waiting period for innovative medications from the current 60 working days to 30 working days in a draft regulation that was issued in Chinese.
An important step in strengthening India's clinical research environment has been taken with the official signing of Memorandums of Agreement (MoAs) by the Indian Council of Medical Research (ICMR) with a number of sponsors under its Network of Phase 1 Clinical Trials. The signing of these agreements is another proof that ICMR has established strong partnerships with significant industry players. It highlights how dedicated the organization is to establishing a robust clinical trial environment in India.
Europe is expected to grow significantly in the humanized mouse model market during the forecast period. Due to increased research facilities and their use in research organizations, especially in the life sciences, the global market is anticipated to grow. Due to advancements in genetic research and a number of active studies employing the humanized mouse model, the global market in European economies is growing. The development of a novel genetically modified human model utilizing state-of-the-art technology is one noteworthy development that positively impacts the global humanized hepatic mouse model.
On July 4, 2024, the Medical Research Act ("Medizinforschungsgesetz") was enacted by the German Parliament as part of a larger national policy to promote pharmaceutical and medical device research and manufacturing in Germany. This new development will surely help trial sponsors in Germany, especially as much speedier contract approval cycles and talks are expected.
New guidelines for performing clinical research have just been implemented in the UK. In December 2024, the updated regulations were initially presented to Parliament with the goal of putting trial participants at the forefront of trial operations and enabling faster, more effective approvals, which will make it easier to test new medications in the UK.
In June 2025, according to Monika Buczek, PhD, Director, Humanized Immune Model Core at Taconic Biosciences, the huSelect portfolio of services was created to provide researchers with the resources they need to carry out dependable, repeatable, and clinically relevant immuno-oncology, autoimmune, and infectious disease studies using humanized immune system mice. Researchers can increase the quality and consistency of their treatment trial results and decrease donor-to-donor variability by using bespoke models with properly chosen CD34+ donor criteria. (Source - Taconic)
By Model Type
By Application
By End User
By Region
February 2026
November 2025
January 2026
November 2025