January 2026

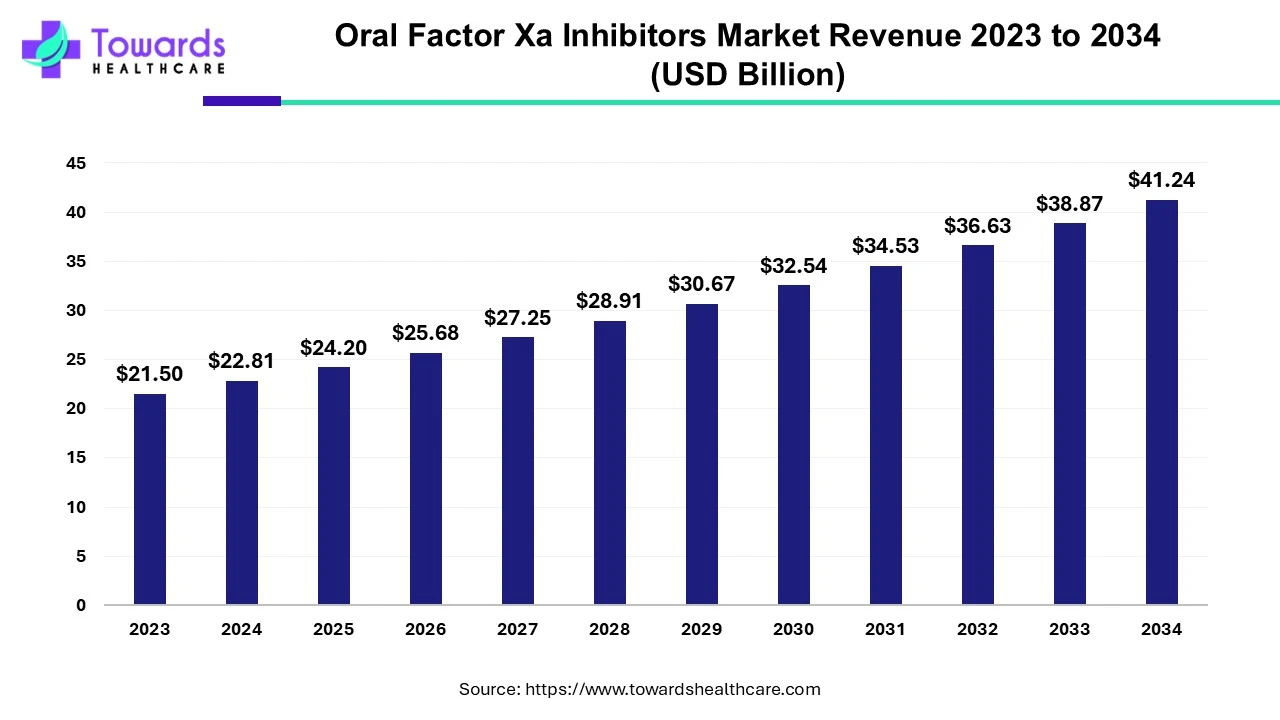
The global oral factor Xa inhibitors market was estimated at US$ 21.5 billion in 2023 and is projected to grow to US$ 41.24 billion by 2034, rising at a compound annual growth rate (CAGR) of 6.1% from 2024 to 2034. The demand for oral factor Xa inhibitors is increasing due to their ease of use. The inhibitors are easy to store and consume and do not require any professionals to administer them.
The oral factor Xa inhibitors market deals with the development and production of various drugs used as anticoagulants. These anticoagulants inhibit Xa factors that prevent blood clotting. Some of the examples of anticoagulants that inhibits Xa factor are apixaban, rivaroxaban, and edoxaban. Oral inhibitors are consumed in the form of tablets or capsules and do not require professionals to administer them, which gives them an advantage over injectables. The demand for these inhibitors is high in the market due to the growing prevalence of various cardiovascular diseases. Cardiovascular disease remains the leading cause of death worldwide. Using anticoagulants helps in preventing emergency or life-threatening situations.
Worldwide, more than 100 million people have had a stroke. More than 60% of strokes occur in adults under 70, and 16% occur in those under 50. By 2030, the entire cost of stroke is predicted to reach US$1 trillion US$, and its economic effect now accounts for 0.66% of the world's GDP. These individuals' risk of ischemic stroke is decreased when the right anticoagulants are used. Finding the optimum antiplatelet and anticoagulant combination in a safe and effective preventative guideline is a major medical objective. Oral direct factor Xa inhibitors have been approved by the U.S. FDA to prevent stroke.
US$3,784.20 was the cost per patient for a 28-day treatment of factor Xa inhibitor. Factor Xa inhibitors for a 28-day course of treatment, including follow-up and per-patient fees, totaling US$77,125.60 dollars. US$4,370.93 was the weighted average cost per patient for a 28-day treatment. In the United States, the yearly cost differential may reach more than US$14.1 billion by 2040. Factor Xa inhibitor thromboprophylaxis can cost up to 1,980.6% more per patiens than an aspirin regimen because of greater main treatment costs, variable PJI rates, and high management expenses.
The market future is promising, driven by the increasing use of artificial intelligence (AI) in developing novel drugs. AI and machine learning (ML) can screen a large number of drug candidates to obtain hits with higher efficacy and lower side effects. They can also predict the pharmacokinetic and pharmacodynamic profiles of novel drugs and suggest modifications of chemical structures accordingly. AI and ML can predict treatment outcomes of oral Factor Xa inhibitors in patients and suggest appropriate dosage. Moreover, government policies for early diagnosis of cardiovascular disorders and stroke allow healthcare professionals to provide advanced treatment, presenting future market growth opportunities.
The North American market is growing strongly due to many factors. These factors include investments in research & development, advanced infrastructure, innovation & growth in the pharmaceutical industry, growing cases of cardiovascular diseases, and government support. Various countries contribute to the growth, among which the U.S. and Canada are the major contributors. These countries have advanced technology, highly skilled professionals, and all the resources needed by key market players and organizations to conduct research, clinical trials, and experiments. This boosts the region, and the organizations are able to invest in other regions as well.
The U.S. held the major share of the oral factor Xa inhibitors market mainly due to resources, a dominant pharmaceutical industry, and the presence of major market players. Another factor that contributed to the market’s growth is the growing prevalence of cardiovascular disease. It is a leading cause of death, and stakeholders are continuously making efforts to mitigate the burden of cardiovascular diseases on the healthcare system.
For instance,
The oral factor Xa inhibitors market is growing significantly due to the growing geriatric population. This group of people is prone to various cardiovascular diseases due to age-related decline in health. Apart from this, various organizations are investing in Asia Pacific’s market due to growing potential in healthcare and pharmaceuticals. Countries like India, China, South Korea, and Japan holds majority of share out of which China is dominant and India is emerging as a potential market place.
There are thought to be 330 million CVD patients in China at the moment. Without health care reform, health spending will rise from USD 543.5 billion in 2014 to USD 2.5 trillion in 2035, according to a joint analysis by the World Bank and the Chinese government. In order to address the issue of growing healthcare expenditures, the Chinese government has initiated significant reforms and implemented significant structural changes. The Chinese government's health and development program places a strong emphasis on creating a "Healthy China," which might have enormous positive effects on the rest of the globe. In the last twenty years, more than ninety-five percent of Chinese people now have health insurance. Due to economic development and increased personal earnings, the nation has been able to raise 600 million people out of poverty during the past three decades.
Europe is considered to be a significantly growing area in the oral factor Xa inhibitors market, due to the rising prevalence of hematological disorders and the increasing number of clinical trials. Research institutions and companies in European countries adopt advanced technologies to develop novel oral factor Xa inhibitors. Government organizations support research activities through funding. They also encourage patients to undergo screening and early diagnosis of hematological disorders.
Annually, 1.1 million people are estimated to be affected by stroke in Europe, and nearly 10 million people are living with the long-term impacts of stroke. The European Union has launched the “Stroke Action Plan for Europe” (SAP-E) for stroke care and support across Europe. As of July 2025, there are 121 clinical trials reported on the clinicaltrials.gov website within 500 miles of Europe related to oral Factor Xa inhibitors as an intervention. (Source: clinicaltrials.gov)
By type, the apixaban segment held a significant share of the market. This segment dominated because apixaban is an oral bioavailable, strong, and highly selective inhibitor. Apixaban is well tolerated and safe; neither the single ascending dosage nor the food impact trial showed any significant bleeding-related events or serious adverse events.
The rivaroxaban segment is estimated to grow at a significant rate during the forecast period. One new oral anticoagulant (NOAC) medication is rivaroxaban. It has a number of off-label and FDA-approved clinical applications. Rivaroxaban is quickly absorbed, peaking in concentration in 2–4 hours, and has a high oral bioavailability (80%).
By application, the atrial fibrillation segment held a significant share of the market and is expected to grow significantly during the predicted period. As the number of patients with atrial fibrillation (AF) keeps rising, it is imperative to avoid strokes in this disease safely and effectively. For this indication, a number of innovative oral anticoagulants are being explored to replace warfarin. Since factor Xa inhibition is known to be a viable target for therapeutic anticoagulation, direct factor Xa inhibitors make up the biggest class of oral anticoagulants now under research.
Mene Pangalos, Executive Vice President of Biopharmaceuticals R&D at AstraZeneca, commented that millions of people depend on FXa inhibitors to prevent harmful blood clots from forming, but also have a risk of acute major bleed. The company is proud to offer the first and only approved treatment to specifically reverse FXa inhibitor activity and help achieve hemostasis, providing an effective and reliable treatment when immediate care is required. (Source: Businesswire)
By Type
By Application
By Region
January 2026
December 2025
November 2025
December 2025