December 2025

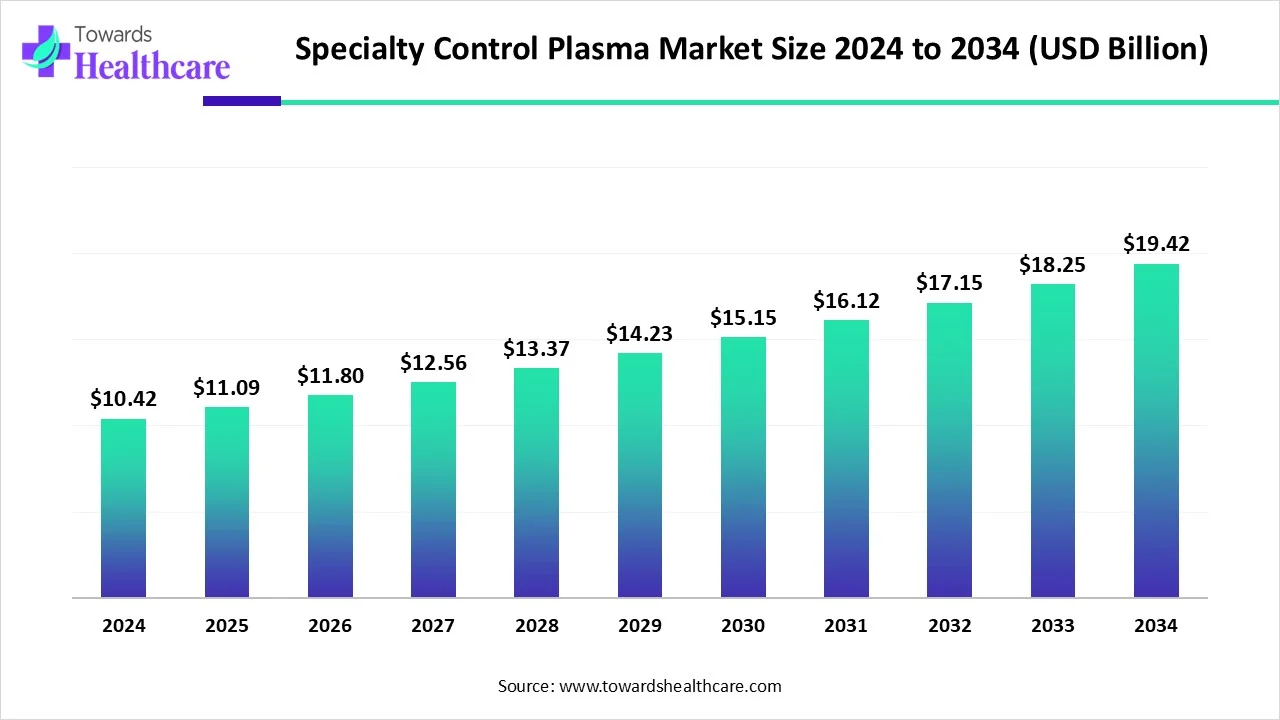
The global specialty control plasma market size is calculated at USD 10.42 in 2024, grew to USD 11.09 billion in 2025, and is projected to reach around USD 19.42 billion by 2034. The market is expanding at a CAGR of 6.44% between 2025 and 2034.
The market for expert control plasma is expanding for several key reasons. The increasing need for accurate and reliable diagnostic testing is driving the need for specialist control plasma, which ensures the quality and uniformity of laboratory tests.The availability and purity of control plasma are increased by technological advancements in plasma collection and processing, which supports the expansion of the sector. Labs and healthcare providers are increasingly realizing the need for quality control in diagnostic testing, which helps the market grow. Specialty control plasma products are also encouraged by regulations that demand high-quality standards in laboratory testing.
| Metric | Details |
| Market Size in 2025 | USD 11.09 Billion |
| Projected Market Size in 2034 | USD 19.42 Billion |
| CAGR (2025 - 2034) | 6.44% |
| Leading Region | North America |
| Market Segmentation | By Type, By Application, By End User, By Region |
| Top Key Players | Precision BioLogic Incorporated, Baxter BioScience, Grifols S.A., Shire Plc., VitroPharma, Kedrion, China Biologic Products (Shandong Taibang), Biotest AG, Sanquin, Talecris Biotherapeutics, SK Plasma, CSL, Octapharma, Cerus |
In clinical diagnostics, specialty control plasma is an essential laboratory product that ensures the precision and consistency of coagulation tests, such as prothrombin time (PT) and activated partial thromboplastin time (aPTT). This control plasma is meticulously prepared to incorporate known levels of clotting factors and is taken from either human or animal sources. It serves as a benchmark for confirming coagulation test findings. Labs can preserve reliable and consistent diagnostic results across various testing platforms by recognizing variations in test results. The specific control plasma. The primary factors propelling market expansion are the aging population, the substantial investment in research and development, and the rising demand for drugs made from plasma globally.
Artificial intelligence (AI) is becoming increasingly important in the field of specialized control of plasma, particularly in medical applications. By making plasma procedures more automated, accurate, and efficient, AI algorithms have the potential to enhance treatment outcomes and our comprehension of plasma-related phenomena. AI is being used to simulate, evaluate, and regulate Cold Atmospheric Plasma (CAP) for a variety of medicinal uses, such as wound healing, cancer treatment, and antimicrobial therapies. AI can help with patient-specific plasma treatment customization by analyzing patient-specific data and modifying therapy parameters for optimal results.
Prevalence of Hemophilia
Around 100% of patients in high-income nations are diagnosed with hemophilia, while only 12% of individuals in low-income countries are diagnosed with the condition. About 1 in 5000 males are thought to have hemophilia, and 1 in 1333 live male newborns are thought to have it. It is possible to diagnose hemophilia patients using specialty control plasma, which aids in the provision of appropriate treatment.
Lack of Plasma Donation
However, the lack of plasma donors is expected to hinder the growth of the specialized control plasma market to some extent throughout the forecast period. People everywhere are impacted by this scarcity, but it is especially apparent in places where there are fewer contributors. Patients who have treatment delays may experience severe health issues or possibly pass away.
Development of Novel Products
The market is also growing as a result of the development of novel control plasma products with improved performance and long-term stability. Finally, the proliferation of remote diagnostics and cloud-based laboratory management systems, especially in developing countries, opens up new market opportunities.
By type, the factor VIII concentrate segment dominated the specialty control plasma market in 2024. Often referred to as antihemophilic factor, factor VIII is a crucial part of the intrinsic clotting cascade. Accurate assessment of clotting factor VIII is necessary for a complete diagnosis and treatment of hemophilia A patients. At this time, five FVIII EHL products are available for clinical use.
By type, the factor IX concentrate segment is expected to grow at the fastest CAGR in the specialty control plasma market during the forecast period. Hemophilia B, or factor IX deficiency, is a little less common bleeding disorder than factor VIII. It is caused by a lack of clotting factor IX. Factor IX deficiency affects 1 in 10,000 live male births worldwide. Patients with severe Factor IX Hemophilia B (less than 1% factor IX) are advised to have preventative factor replacement therapy in order to avoid severe spontaneous bleeding.
By application, the coagulation disorders segment led the specialty control plasma market in 2024. Even though they are uncommon, these conditions can cause severe bleeding; therefore, early identification is crucial for selecting the best course of treatment. In affluent nations, cutting-edge therapeutic alternatives are starting to appear, and knowledge of the prevalence, clinical manifestations, and management techniques of these illnesses has been established. Understanding the prevalence of these conditions would aid in the establishment of facilities for early identification and suitable treatment, therefore averting potentially fatal bleeding and death.
By application, the hypogammaglobulinemia segment is expected to grow at the fastest CAGR in the specialty control plasma market during the forecast period. A laboratory diagnostic known as hypogammaglobulinemia is characterized by serum IgG levels that are below normal for the patient's age. Regular follow-up is necessary for patients with hypogammaglobulinemia of uncertain importance and origin in order to receive additional diagnosis and treatment.
By end-user, the hospitals segment dominated the specialty control plasma market in 2024. As the healthcare sector continues to change, multispecialty hospitals are establishing diagnostic services to give a range of institutions access to cutting-edge medical equipment, technology, and gadgets. It is also folks who get the fastest outcomes. Multispecialty hospitals offer a wide range of medical specializations, diagnostic services, and emergency care in your area.
By end-user, the clinics segment is expected to grow at the fastest CAGR during the forecast period. Clinics are crucial to the contemporary healthcare system because they can diagnose a wide range of disorders quickly and accurately. These facilities employ a variety of state-of-the-art diagnostic techniques and instruments to help physicians identify the underlying causes of diseases, monitor their progression, and develop effective treatment plans.
North America dominated the specialty control plasma market in 2024, mostly as a result of the area's highly developed industrial and development capacities. There are plenty of growth prospects due to the existence of important industry players in the U.S. and Canada. In the meantime, the prevalence of chronic illnesses is rising along with improvements in medical technology. Further driving market expansion in this area are rising awareness of plasma donation and the increasing need for medicines given by plasma.
One main immunodeficiency disorder affects at least one in every 2,000 Americans. The exact incidence is unclear, although estimates of PID patients worldwide range from 390,000 to 6 million. In the U.S. alone, the incidence is thought to be as high as 50.5 instances per 100,000 people. Even while severe versions are more prevalent in infancy and early childhood, the illness may not manifest symptoms in other situations until later in life. Antibody deficiency is the cause of almost half (63.4%) of PID cases in the U.S.
More than 35,000 of them have symptoms of blled condition that are severe enough to require medical care. Thanks to the Hemophilia Research Million Dollar Club endowment, kind individual donors, committed corporate sponsors, and CHS chapters and regions across the country, the CHS has been able to invest over $10 million in research in Canada for over 30 years.
Asia Pacific is estimated to host the fastest-growing specialty control plasma market during the forecast period. Driven by advancements in medical technology and the rising prevalence of chronic disorders. Growing awareness of plasma donation and the growing need for medications delivered by plasma are further factors propelling market growth in this sector. It is also driven by greater financing for medical care, more diagnostic testing, and a better understanding of blood disorders. Because of the growth of labs, advancements in healthcare, and the large number of patients with chronic diseases who need routine blood testing, specialty plasma is in high demand in China, Japan, and India. The expansion of this sector is also aided by government programs to improve lab certification and standards.
Over 65,000 individuals with hemophilia were predicted to exist in China, a vast nation with a population of 1.41 billion. For four decades, China has worked to enhance the treatment of hemophilia. Over time, the Chinese Hemophilia Treatment Center Collaborative Network came into being.
The Haemophilia and Health Collective of North (HHCN) estimates that India has the second-highest number of hemophilia patients globally, with an estimated 1,36,000 cases of hemophilia A. The high incidence of hemophilia in India makes it necessary to attend to the special needs of those who are affected.
Europe is expected to grow significantly in the specialty control plasma market during the forecast period. As a result of rising blood clot rates, stricter clinical testing regulations, and a greater need for accurate laboratory testing. According to the In Vitro Diagnostic Regulation (IVDR) and the European Medicines Agency (EMA), specialty control plasma products must be safe and comply with certain rules. The market's growth is mostly attributed to the region's leading countries. This is because they have strong testing facilities, spend a lot of money on research, and use external quality assurance (EQA) programs more often. Additionally, growing awareness of early blood illness surveillance and identification is driving market growth.
Hemophilia affects approximately 6,000 people in Germany. About 150 patients with severe coagulation problems are treated over the long term at the MHH's Hemophilia Center. 76% of German doctors said they had experienced at least one bleeding event in the previous 12 months.
In 2023, von Willebrand disease was found in 12,077 individuals in the UK, while other bleeding disorders were detected in 15,265 individuals. In the UK, 7,625 women and 4,310 men have been diagnosed with von Willebrand disease (VWD), which is the most common and somewhat more common in women than in men.

In February 2024, because disorders like CAD and PAD can have serious consequences, there is an urgent need for better detection and treatment. In the world, more than 200 million people have PAD, and CAD alone accounts for around one in five deaths. These numbers, together with our growing understanding of these conditions, are driving a sharp rise in the demand for direct oral anticoagulants globally, according to Frank Sieben, Head of Core Lab Customer Area at Roche Diagnostics. (Source - Roche)
By Type
By Application
By End User
By Region
December 2025
October 2025
November 2025
November 2025