
Image Credits: Chosunbiz
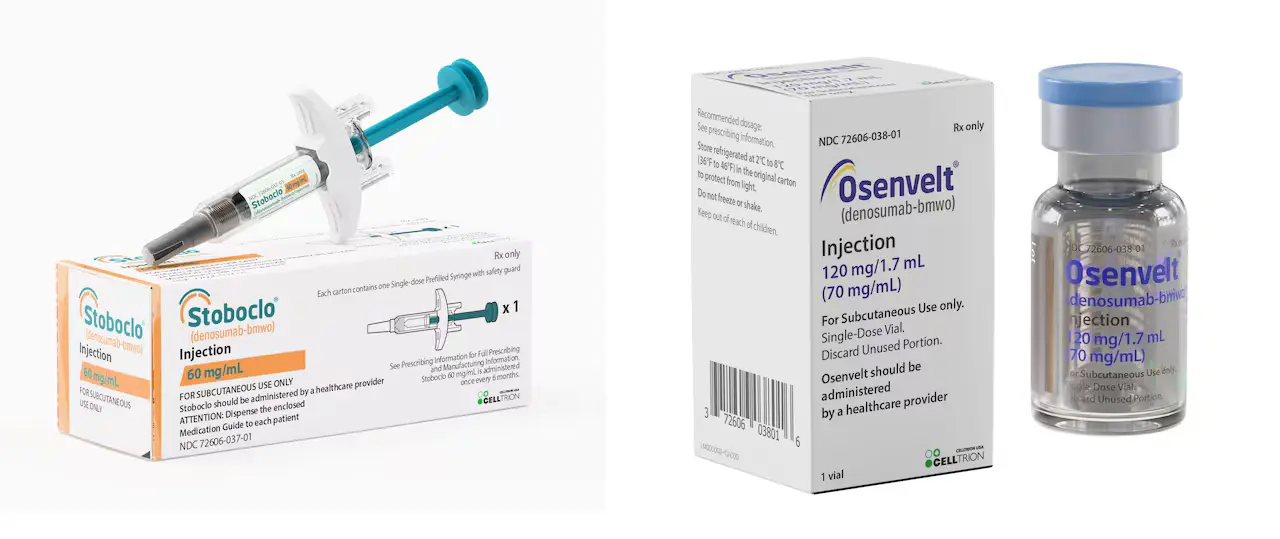
Celltrion USA confirmed that OSENVELT (denosumab-bmwo) and STOBOCLO (denosumab-bmwo), references to XGEVA and PROLIA, are available in the US. Access to these medications will improve and enhance patient care. This is a new solution to the healthcare market. Celltrion has supported OSENVELT and STOBOCLO through its patient support program. This program is introduced to encourage patients to lead their treatment journey more trustfully. Celltrion provides several resources, including Celltrion’s CARES Co-pay assistance program and Celltrion’s CONNECT Patient support program. Celltrion’s other biosimilar medication (treatment) portfolio has also covered the areas of gastroenterology, endocrinology, immunology, allergy, and oncology.
OSENVELT® with 120 mg/1.7 mL (70 mg/mL) injection is available in the clinics and hospitals. This injection is for patients suffering from bone metastases from solid tumors and various myeloma conditions, which are said to be skeletal-related events. It is a treatment that treats skeletally mature adolescents and adults having huge cell tumors of bone. The surgical resection is a common result in extreme morbidity; it also cures hypercalcemia of malignancy refractory to bisphosphonate therapy.
STOBOCLO® with 60mg/ml injection is available for postmenopausal womens. This is a treatment for postmenopausal women with osteoporosis that can reduce the risk of fracture. This injection increases and improves bone mass in men having osteoporosis, which treats the glucocorticoid-induced osteoporosis in both women and men to avoid fractures. This will allow an individual to receive androgen deprivation therapy for nonmetastatic prostate cancer. The similar risk of fracture can be avoided by receiving adjuvant aromatase inhibitor therapy for breast cancer.
The chronic kidney disease-mineral bone disorder (CKD-MBD) causes a high risk of hypocalcemia in these patients. Before taking STOBOCLO® (denosumab-bmwo) for advanced chronic kidney disease, patients should consider supervision by a healthcare professional who is experienced in managing and diagnosing CKD-MBD. STOBOCLO is not suitable for pregnant women, hypocalcemia, and hypersensitive patients. The direction and indication vary for every individual and should be taken under consultation by healthcare professionals.
Chief Commercial Officer at Celltrion US, Thomas Nusbickel, said, “We are proud of our achievement in settling globally with Amgen for denosumab biosimilars. We are excited about the launch of our denosumab biosimilars in the US market for healthcare professionals and patients by enabling various alternative treatment options. Strengthening our heritage in biosimilars, Celltrion continues with its commitment to stay a trustworthy partner for physicians and patients, further supporting and promoting sustainability to healthcare systems.”